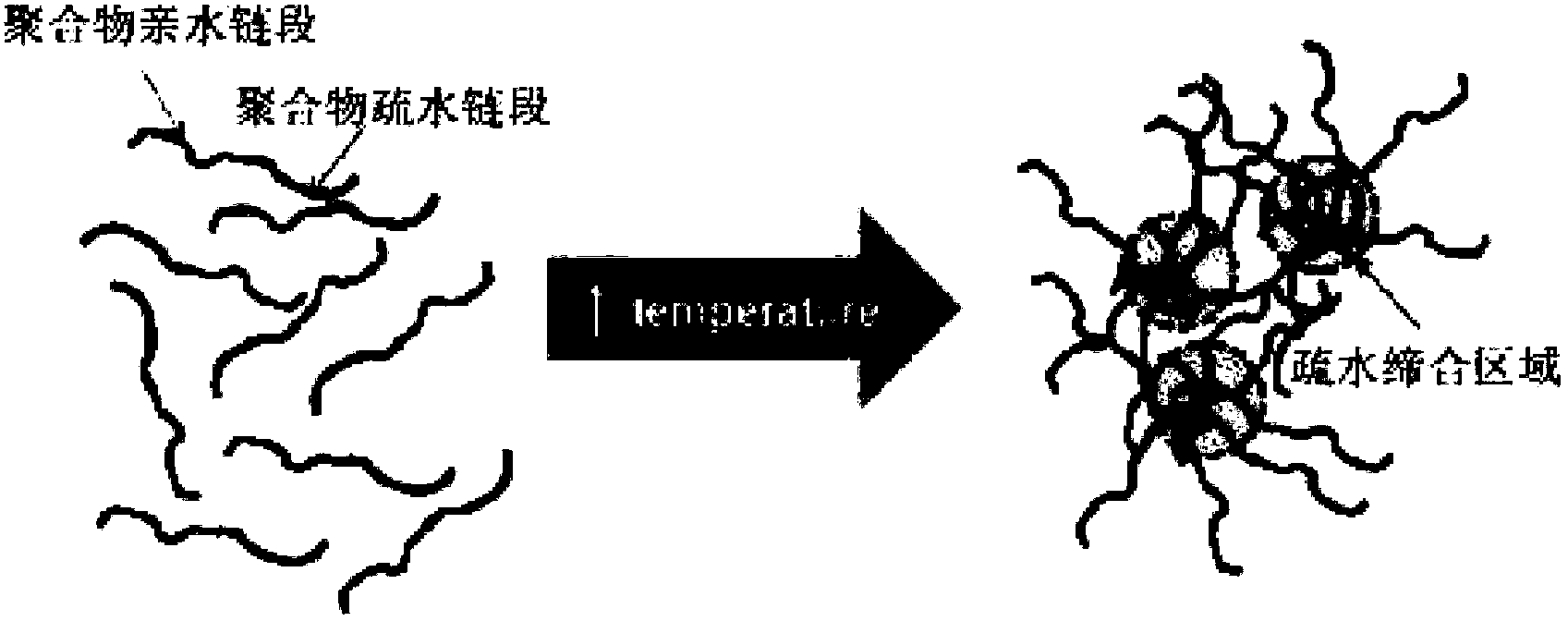
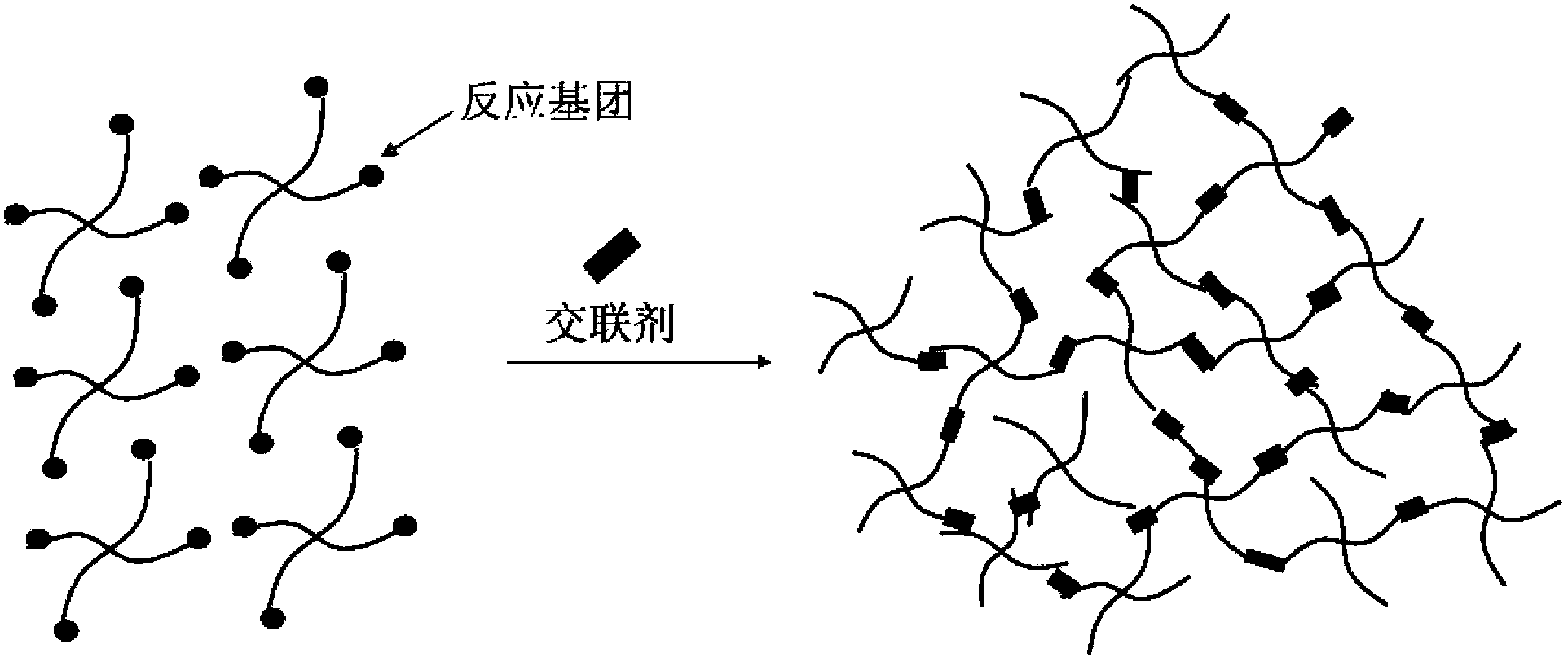
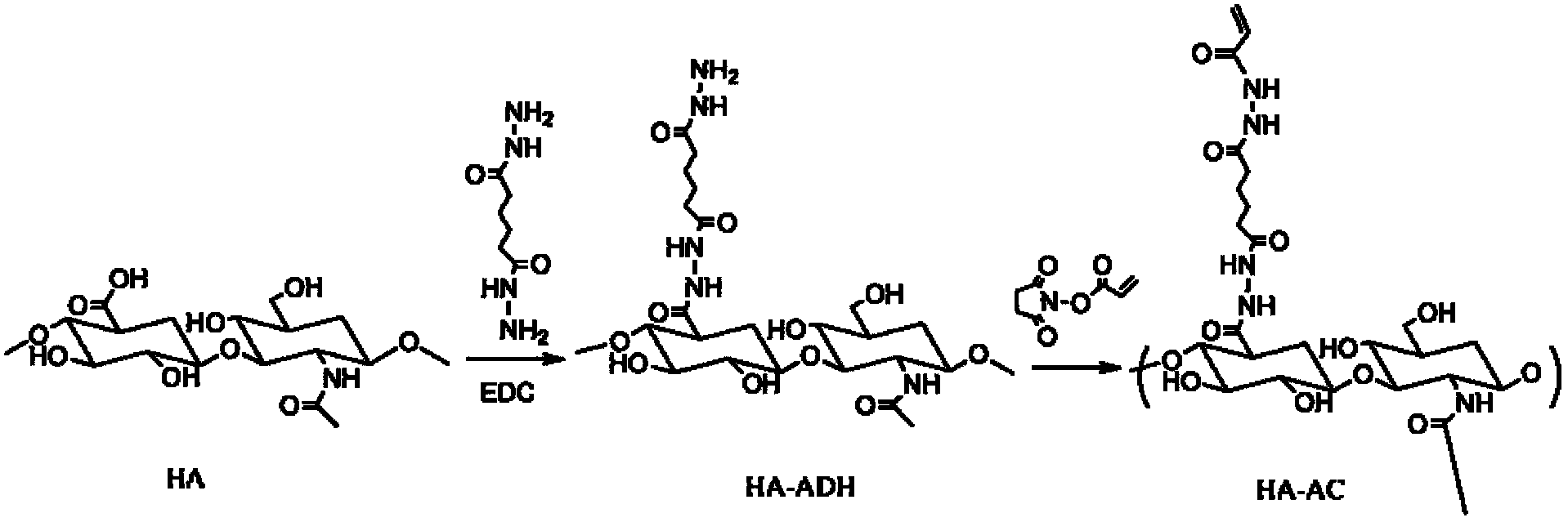
Preparation method and application of injectable hyaluronic acid hydrogel
A technology of hyaluronic acid and hydrogel, which is applied in the field of preparation of injectable hydrogel, which can solve the potential safety hazards of biocompatibility, biodegradability and immunogenicity, low biodegradability efficiency and complex synthesis process and other issues, to achieve the effect of excellent biocompatibility and biodegradability, excellent stability and mechanical properties, and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A method for preparing an injectable hyaluronic acid hydrogel constructed by Michael addition reaction, the specific steps are as follows:
[0046] 1) Preparation of hyaluronic acid-adipate dihydrazide derivative (HA-ADH)
[0047] Put 2g HA (molecular weight 50KDa) solution in 400mL deionized water, then add 20g adipic acid dihydrazide and adjust the pH value of the solution to 5.0-7.4 with hydrochloric acid, then add 4.0g 1-ethyl-(3-dimethylaminopropyl base) carbodiimide (EDC) and reacted overnight. Finally, the product HA-ADH solution is purified by dialysis (molecular weight cut-off 8-10KDa dialysis bag), and then freeze-dried and stored.
[0048] 2) Preparation of acrylate functionalized hyaluronic acid (HA-AC)
[0049] Dissolve 1.9g of HA-ADH prepared in step 1 in 350mL of buffer solution with pH=7.4, and dissolve another 1.33g of N-acryloxysuccinimide (NAS) in 10-20mL of DMSO, and further dilute the NAS solution Add it into the HA-ADH solution, stir overnight a...
Embodiment 2
[0057] A method for preparing an injectable hyaluronic acid hydrogel constructed by Michael addition reaction, the specific steps are as follows:
[0058] 1) Preparation of hyaluronic acid-adipate dihydrazide derivative (HA-ADH)
[0059] Put 2g HA (molecular weight 50KDa) solution in 400mL deionized water, then add 30g adipic acid dihydrazide and adjust the pH value of the solution to 5.0-7.4 with hydrochloric acid, further add 6g 1-ethyl-(3-dimethylaminopropyl ) carbodiimide (EDC), reacted overnight. Finally, the product HA-ADH solution is purified by dialysis (molecular weight cut-off 8-10KDa dialysis bag), and then freeze-dried and stored.
[0060] 2) Preparation of acrylate functionalized hyaluronic acid (HA-AC)
[0061] Dissolve 1.9g of HA-ADH prepared in step 1 in 350mL of buffer solution with pH=7.4, and dissolve another 2.85g of N-acryloxysuccinimide (NAS) in 10-20mL of DMSO, and further dilute the NAS solution Add it into the HA-ADH solution, stir overnight at room...
Embodiment 3
[0067] A method for preparing an injectable hyaluronic acid hydrogel constructed by Michael addition reaction, the specific steps are as follows:
[0068] 1) Preparation of hyaluronic acid-adipate dihydrazide derivative (HA-ADH)
[0069] Put 4g HA (molecular weight 50KDa) solution in 400mL deionized water, then add 40g adipic acid dihydrazide and adjust the pH value of the solution to 5.0-7.4 with hydrochloric acid, then add 4.0g 1-ethyl-(3-dimethylaminopropyl base) carbodiimide (EDC) and reacted overnight. Finally, the product HA-ADH solution is purified by dialysis (molecular weight cut-off 8-10KDa dialysis bag), and then freeze-dried and stored.
[0070] 2) Preparation of acrylate functionalized hyaluronic acid (HA-AC)
[0071] Dissolve 3.85g of HA-ADH prepared in step 1 in 350mL of buffer solution with pH=7.4, and dissolve another 1.92g of N-acryloxysuccinimide (NAS) in 10-20mL of DMSO, and further dissolve NAS The solution was added to the HA-ADH solution, stirred over...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com