Dual sensitive amphiphilic triblock copolymer containing disulfide bond and acylhydrazone bond and preparation method and application of dual sensitive amphiphilic triblock copolymer
A dual-sensitive, disulfide bond technology, applied in the direction of medical preparations, drug combinations, and pharmaceutical formulations of non-active ingredients, can solve the problems of poor controllability and slow drug release, so as to promote rapid release and achieve selectivity Unleashes, enhances healing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Synthesis of dual sensitive amphiphilic triblock copolymers containing disulfide bonds and acylhydrazone bonds
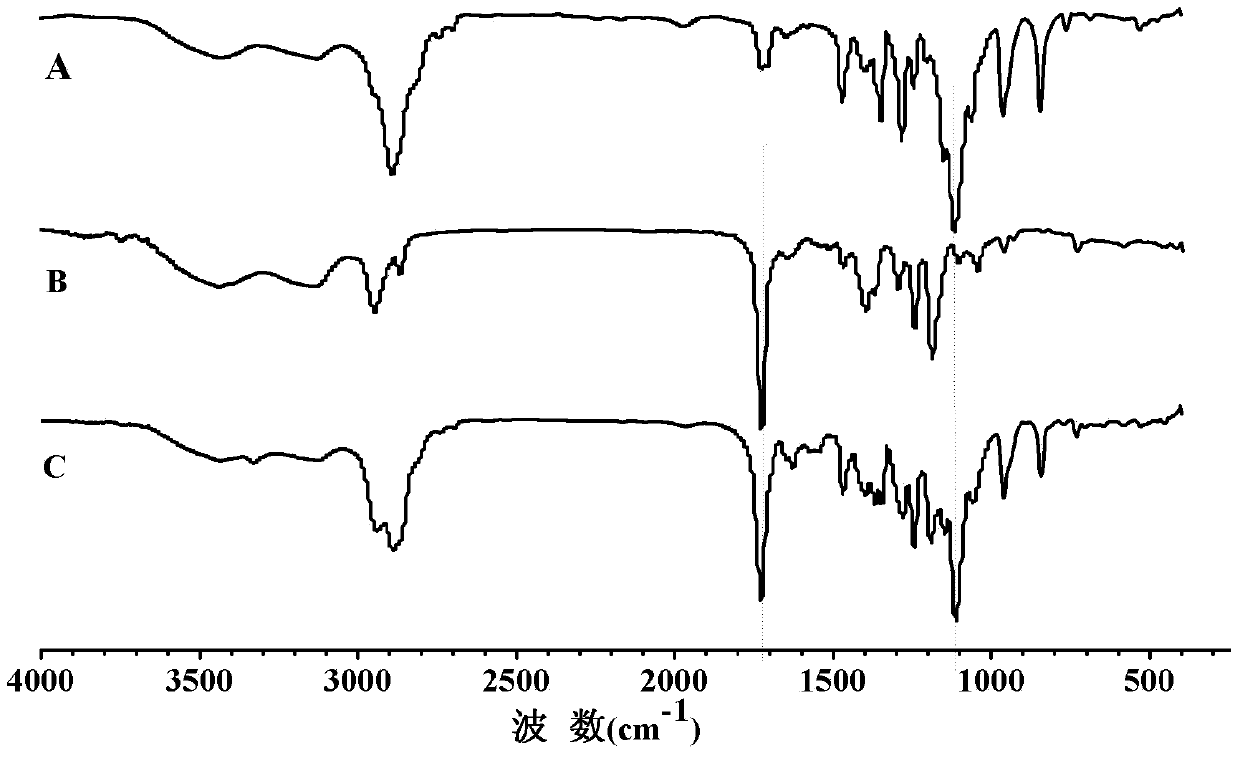
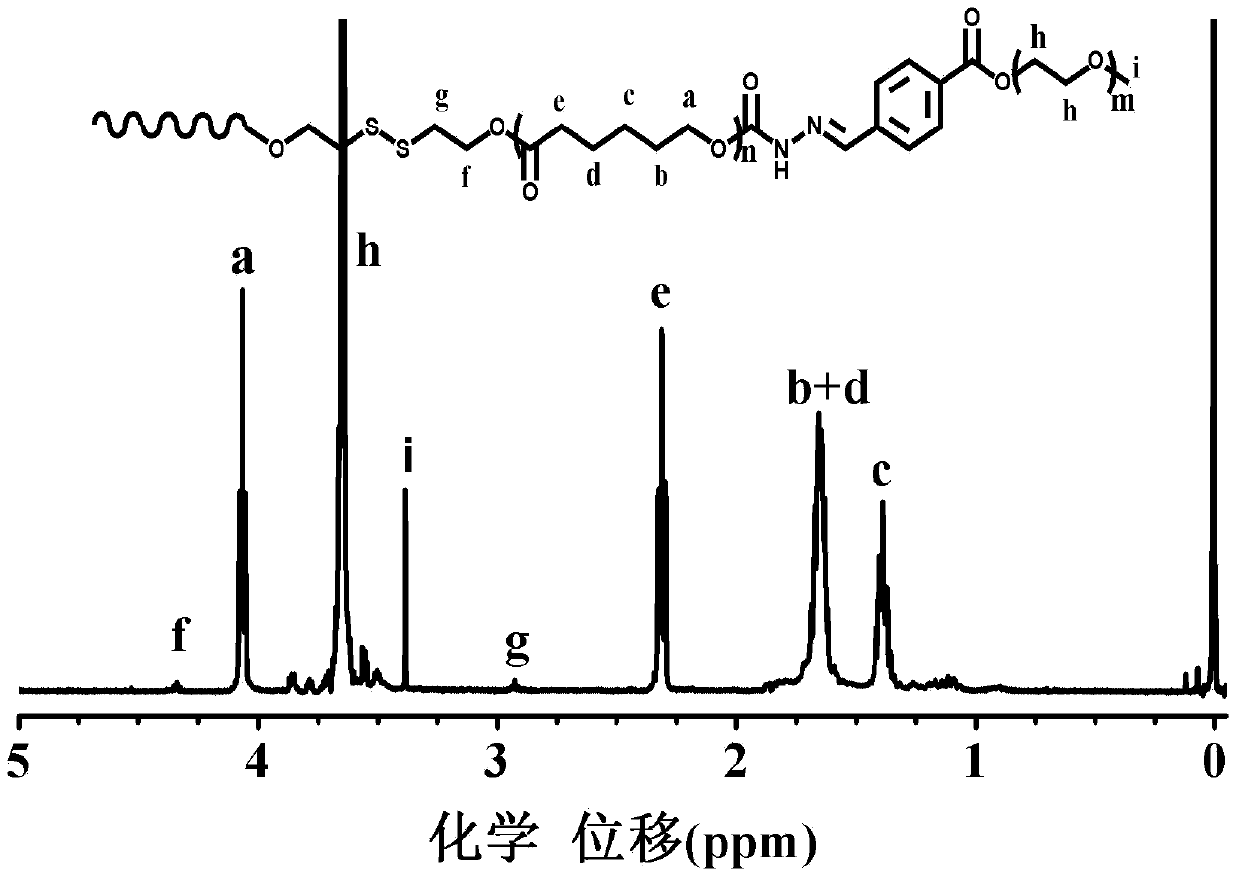
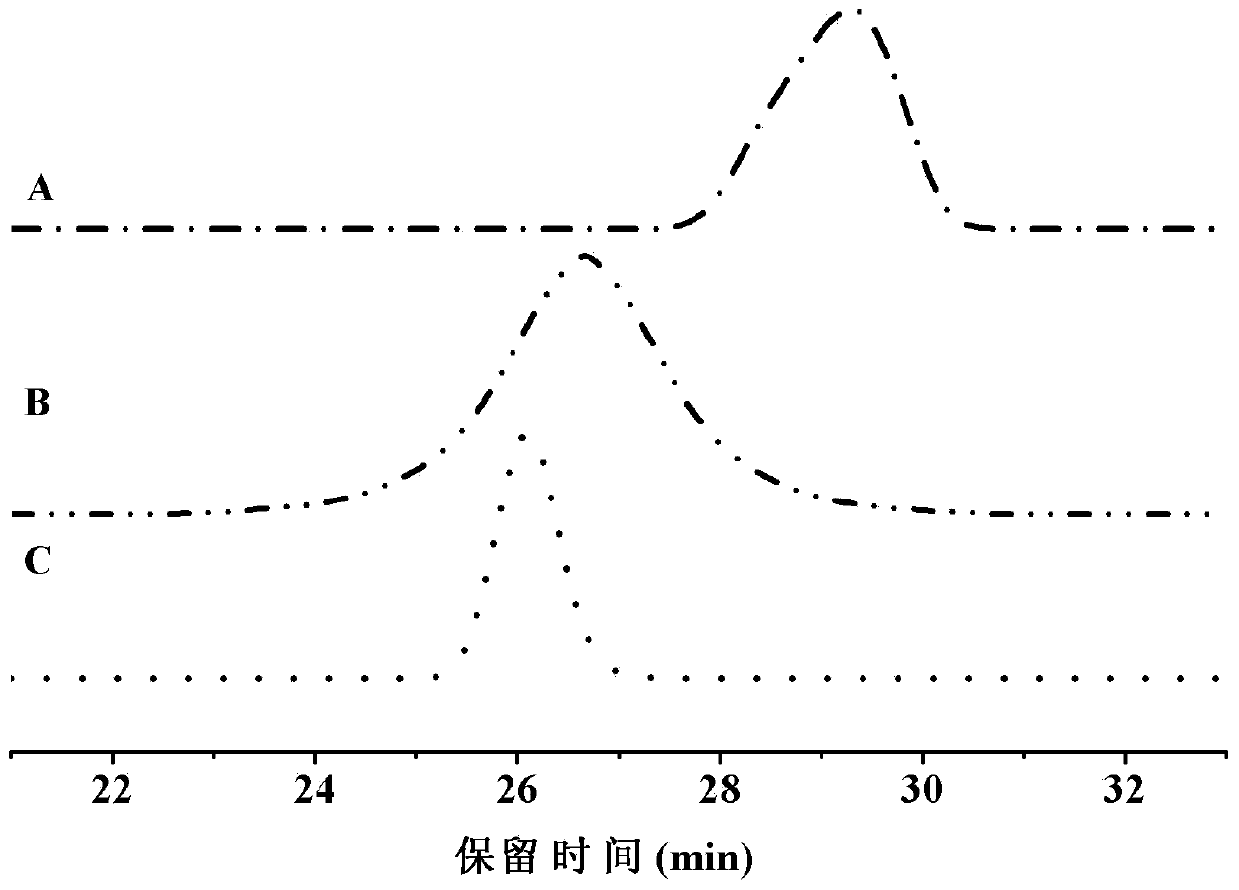
[0029] Dissolve 2 g of disulfide bond-containing polyester in dichloromethane, add 1.62 g of N,N'-carbonyldiimidazole, complete the reaction at room temperature, concentrate, precipitate in glacial ether, filter with suction, and dry in vacuo to obtain activated disulfide-bond-containing polyester; 2 g of activated disulfide-bond-containing polyester, 50 mL of methanol and 0.49 g of hydrazine hydrate were sequentially added to the reactor, and refluxed at 70 ° C. After the reaction was completed, it was dialyzed with water and frozen Dry to obtain the polyester containing active terminal amine groups and disulfide bonds, that is, block A, and its infrared spectrum is as follows: figure 1 As shown in (B), the GPC spectrum is as follows image 3 Shown in (B); With the polyester of 1g and the polyethylene glycol monomethyl ether benzaldehyde ester (i...
Embodiment 2~ Embodiment 10
[0031] The device and operation are the same as in Example 1, except that the composition and relative molecular weight of polyethylene glycol monomethyl ether benzaldehyde ester and disulfide bond-containing polyester are changed to the data in Table 1, and the ratio shown in Table 1 is fed. Other triblock copolymers shown in Table 1 were prepared.
[0032] Table 1 Redox and pH dual sensitive amphiphilic triblock copolymers
[0033]
[0034]
[0035] B The relative molecular mass of polyethylene glycol monomethyl ether benzaldehyde; A Represents the relative molecular mass of the polyester containing disulfide bonds; CL represents caprolactone, which is the monomer shown in formula II; GA stands for glycolide, which is the monomer shown in formula III; LA stands for lactide, which is the monomer shown in formula IV represents the monomer; T represents 1,4,8-trioxaspiro[4.6]-9-undecanone, which is the monomer represented by formula V.
Embodiment 11
[0036]Embodiment 11: device and operation are the same as embodiment 1~embodiment 10, just change wherein polyethylene glycol monomethyl ether benzaldehyde ester into the benzaldehyde of monomethyl-terminated polyoxyethylene and polyoxypropylene block copolymer ester.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com