Polymeric micelle having hydrophilic and hydrophobic terminals and having pH response, preparation and application thereof
A polymer gel, hydrophilic and hydrophobic technology, applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, microcapsules, etc., can solve the problem that the polymer micelle carrier does not achieve satisfactory performance, etc. To achieve the effect of improving stability, drug loading capacity and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
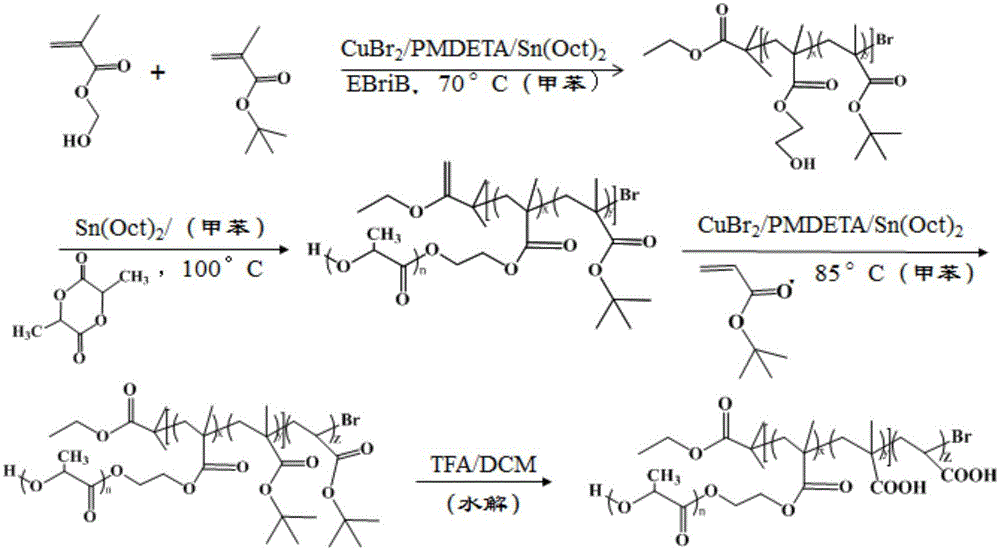
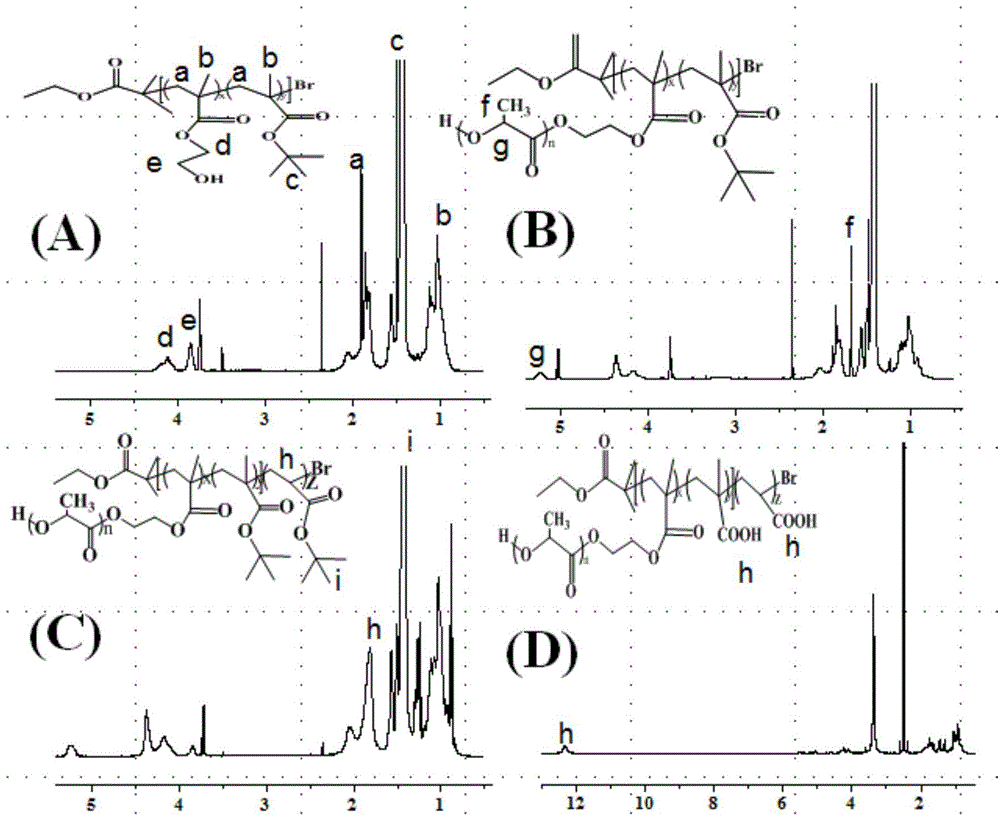
[0086] (1) Synthesis of P(HEMA-co-tBMA)-Br (A:B=14:83, A represents the terminal hydroxyl group HEMA, B represents the pH response group tBMA, the ratio is mass percent, the same below)
[0087] Weigh CuBr 2 (11.2mg) was placed in a 50mL eggplant-shaped reaction bottle with a stirrer, sealed and vacuumed-nitrogen 3 times. Under the protection of nitrogen, sequentially add toluene (10mL), HEMA (1.3mL), tBMA (9.5mL) and ligand PMDETA (105μL) into the reaction flask with a syringe and a pipette gun, and stir for 10min to make the catalyst complex Formation; then the reducing agent Sn(Oct) 2 (202.5 mg) was added to the reaction flask, after stirring for 5 min, the initiator EBriB (150 μL) was added, transferred to a 70° C. oil bath, and stirred and reacted for 3 h under nitrogen protection; after the reaction was completed, cooled to room temperature, added 50 mL THF to dilute, and then Use THF as eluent, filter through neutral alumina column to remove catalyst CuBr 2 , until a...
Embodiment 2
[0109] (1) Synthesis of P(HEA-co-tBMA)-Br (A:B=6:92, A represents the terminal hydroxyl group HEA, B represents the pH response group tBMA)
[0110] Weigh CuBr 2 (11.2mg) was placed in a 50mL eggplant-shaped reaction bottle with a stirrer, sealed and vacuumed-nitrogen 3 times. Under the protection of nitrogen, anisole (10mL), HEA (1.0mL), tBMA (19.3mL) and ligand bpy (187.4mg) were added to the reaction flask with a syringe and a pipette gun in turn, and stirred for 10min to allow Catalyst complex formation. Then the reducing agent Sn(Oct) 2 (268mg) was added to the reaction flask, after stirring for 5min, the initiator EBriB (150μL) was added, transferred to a 70°C oil bath, stirred and reacted for 3h under nitrogen protection; after the reaction was completed, cooled to room temperature, diluted with 50mLTHF, and then THF was used as the eluent, and the catalyst CuBr was removed by filtration through a neutral alumina column. 2 , until a colorless and transparent solutio...
Embodiment 3
[0130] (1) Synthesis of P(HEMA-co-tBA)-Br (A:B=9:72, A represents the terminal hydroxyl group HEMA, B represents the pH response group tBA, the ratio is the mass percentage, the same below)
[0131] Weigh CuBr 2(26.4mg) was placed in a 50mL eggplant-shaped reaction bottle with a stirring bar, sealed and vacuumed-nitrogen three times; under the protection of nitrogen, toluene (10mL), HEMA (0.84 mL), tBA (8.24mL) and ligand PMDETA (248μL) were added to the reaction flask, stirred for 10min to form the catalyst complex; then the reducing agent Sn(Oct) 2 (405mg) was added to the reaction flask, and after stirring for 5min, the initiator EBriB (450μL) was added, transferred to a 70°C oil bath, and stirred and reacted for 3h under nitrogen protection; after the reaction was completed, cooled to room temperature, diluted with 50mLTHF, and then THF was used as the eluent, and the catalyst CuBr was removed by filtration through a neutral alumina column. 2 , until a colorless and tran...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com