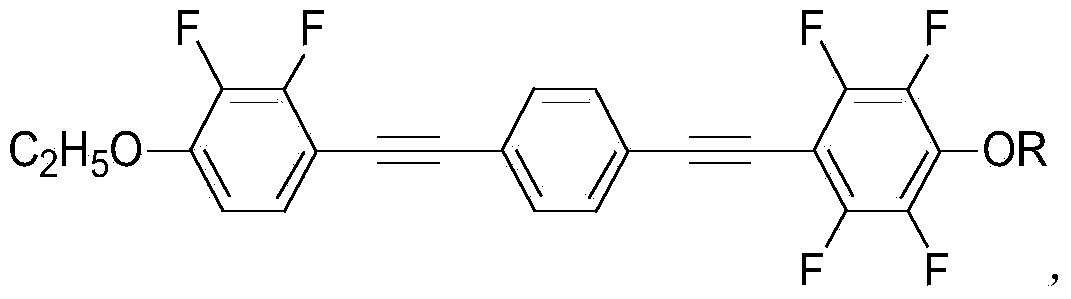
Fluorine-containing diphenyldiacetylene nematic negative liquid crystal, synthesis method and application
A technology of negative liquid crystal and synthesis method, which is applied in the direction of liquid crystal materials, chemical instruments and methods, and preparation of organic compounds, etc. It can solve the problems of poor chemical stability, high melting point, high viscosity, etc., and achieve low viscosity and high chemical stability , the effect of high refractive index difference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
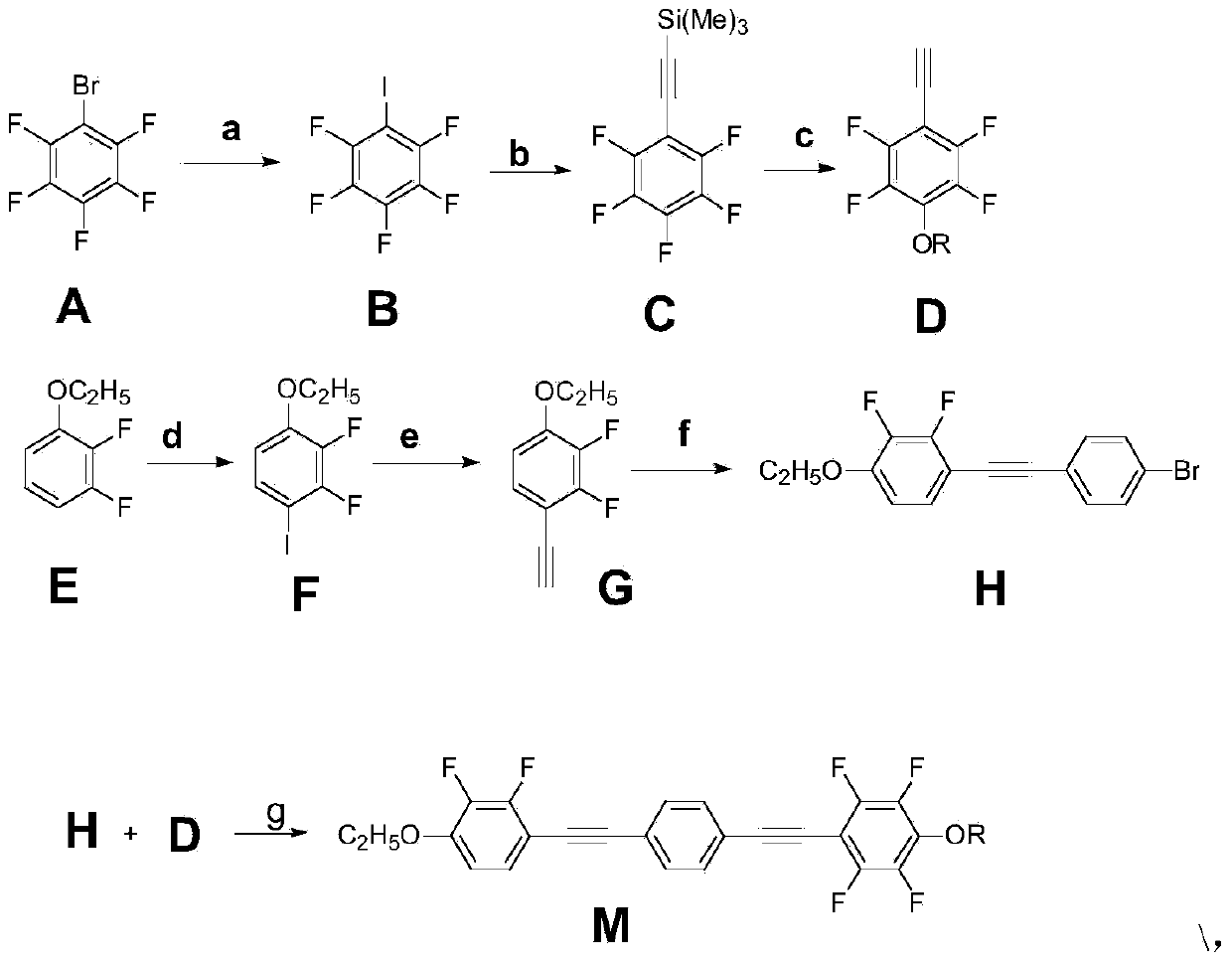
[0031] Example 1: Using 4-[(4-n-propoxy-2,3,5,6-tetrafluorophenyl)ethynyl]-4-[ethoxy-2,3-difluorophenyl)acetylene The synthesis of base] benzene is an example:
[0032] Preparation of pentafluoroiodobenzene
[0033] Under nitrogen protection, in a 250ml three-necked flask, add 6.0g (0.25mol) of magnesium and 100ml of tetrahydrofuran (THF), feed nitrogen for 5min, slowly dropwise add 49.4g (0.2mol) pentafluorobromobenzene and The mixed solution of 70ml THF reacted vigorously. The reaction temperature was controlled by an ice-salt bath at about 0°C, and the dropwise addition was completed in about 40 minutes. The stirring reaction was continued for 1 hour at this temperature. Weigh 50.8g (0.2mol) of iodine and dissolve it in 100ml THF, add it dropwise to the reaction solution, still control the reaction temperature at 0°C, drop it in one hour, continue to react for one hour, and the reaction is complete.
[0034] Add 150ml of dilute hydrochloric acid solution to the reaction s...
Embodiment 2
[0061] The synthetic method of embodiment 2 similar target compound is with embodiment:
[0062] Among them, at room temperature and in DMF solvent, the molar ratio of perfluorophenyltrimethylsilylacetylene D, potassium carbonate and n-alkyl (C1-C8) alcohol is 1:1~2:1~5, and the reaction is 72h . Then continue to react with embodiment 1, obtain result as follows:
[0063] 4-[(4-n-butoxy-2,3,5,6-tetrafluorophenyl)ethynyl]-4-[ethoxy-2,3-difluorophenyl)ethynyl]benzene MS( m / z, %): 502.1 (M+, 42.21), 418.0 (100.00)
[0064] 1 H NMR (400MHz, Chloroform-d) δ7.57–7.50(m,4H),7.23–7.16(m,1H),6.71(ddd,J=9.0,7.4,1.8Hz,1H),4.31–4.25(m ,2H),4.15(q,J=7.0Hz,2H),1.81–1.73(m,2H),1.55(d,J=8.6Hz,2H),1.49(d,J=7.0Hz,3H),0.98 (t,J=7.4Hz,3H).
[0065] Polarizing microscope observation: Cr 109.9°C N 233.6°C I 222.5°C N 95.8°C Cr
[0066] DSC detection data: Cr 98.92°C N230.45°C I 231.51°C N107.79°C Cr.
[0067] 4-[(4-ethoxy-2,3,5,6-tetrafluorophenyl)ethynyl]-4-[ethoxy-2,3-difluorophenyl)ethyn...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com