Brain-targeted water soluble drug carrier as well as preparation method and application thereof
A water-soluble drug and brain-targeted technology, which can be used in drug combinations and neurological diseases, etc., can solve the problems of low protein yield, destruction of LDL structure, and cumbersome procedures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
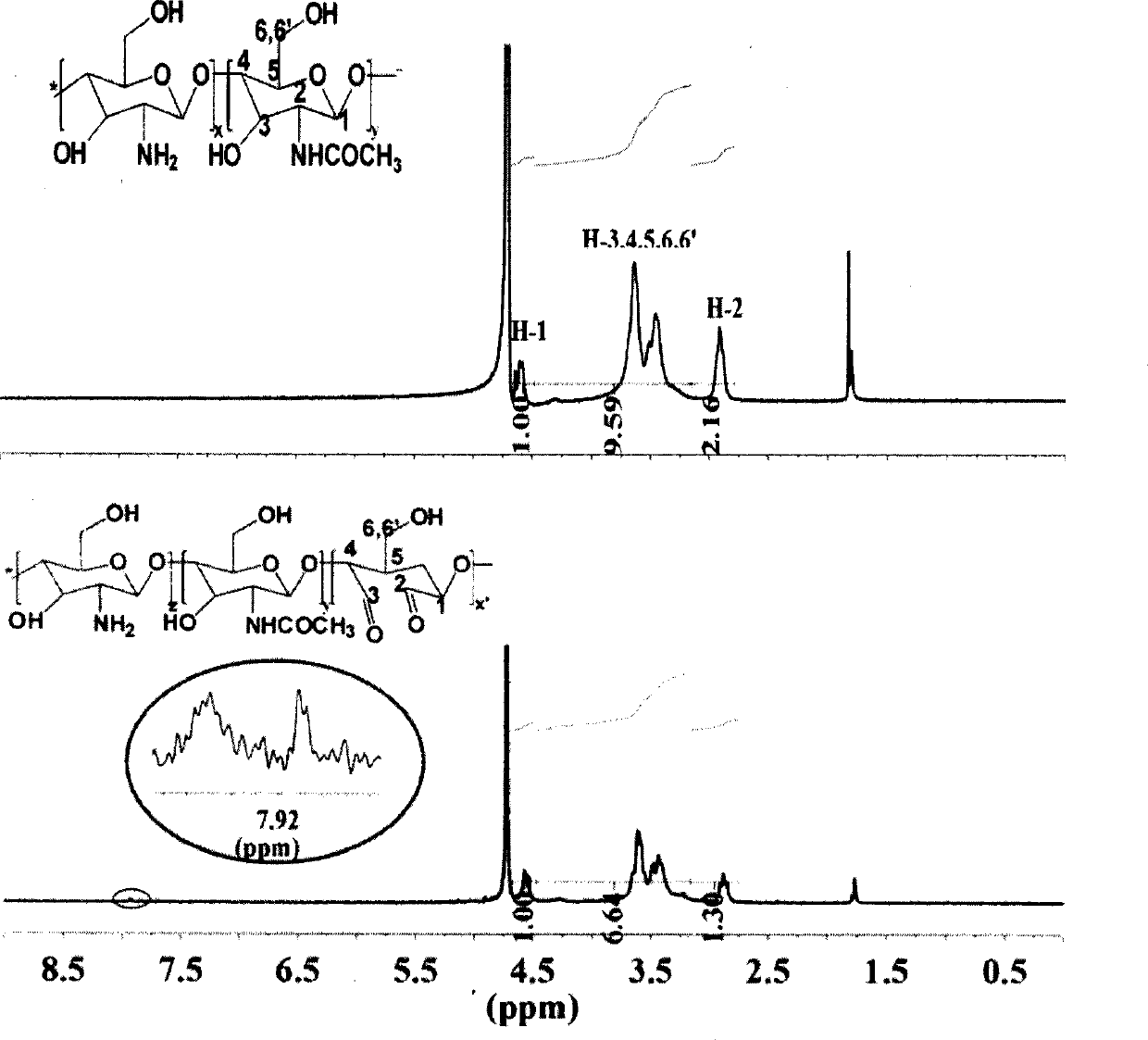
[0048] 1. Synthesis of formaldehyde chitosan (CS-CHO)
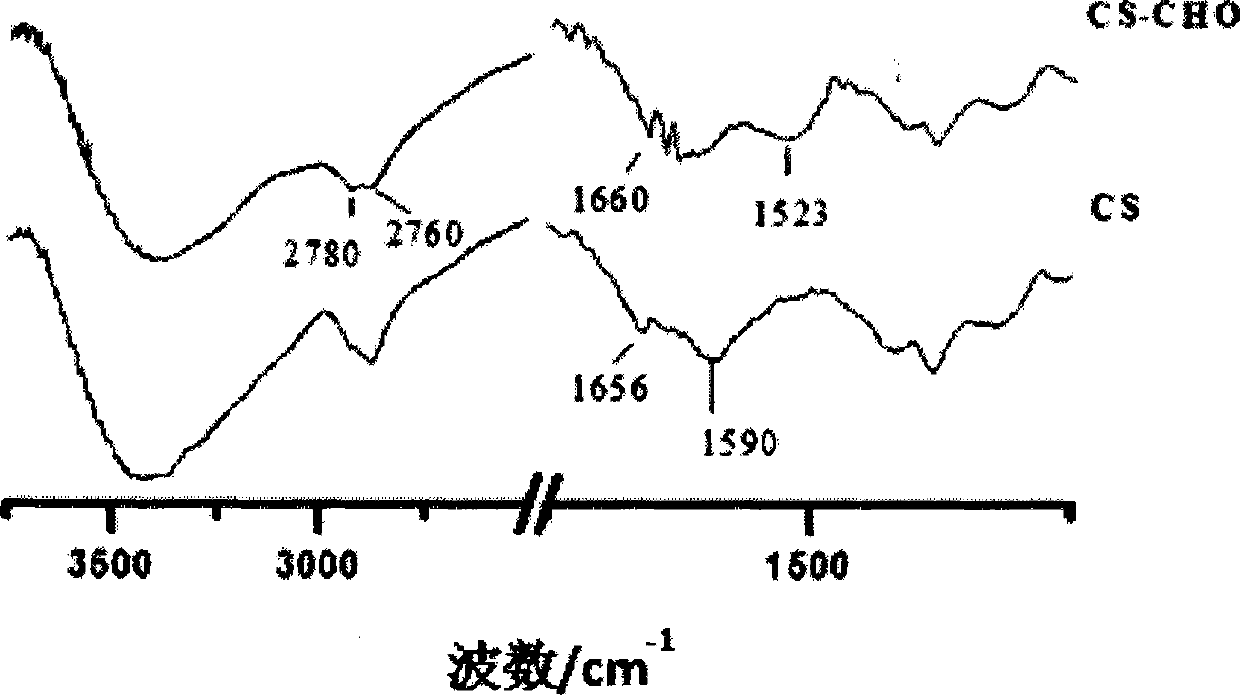
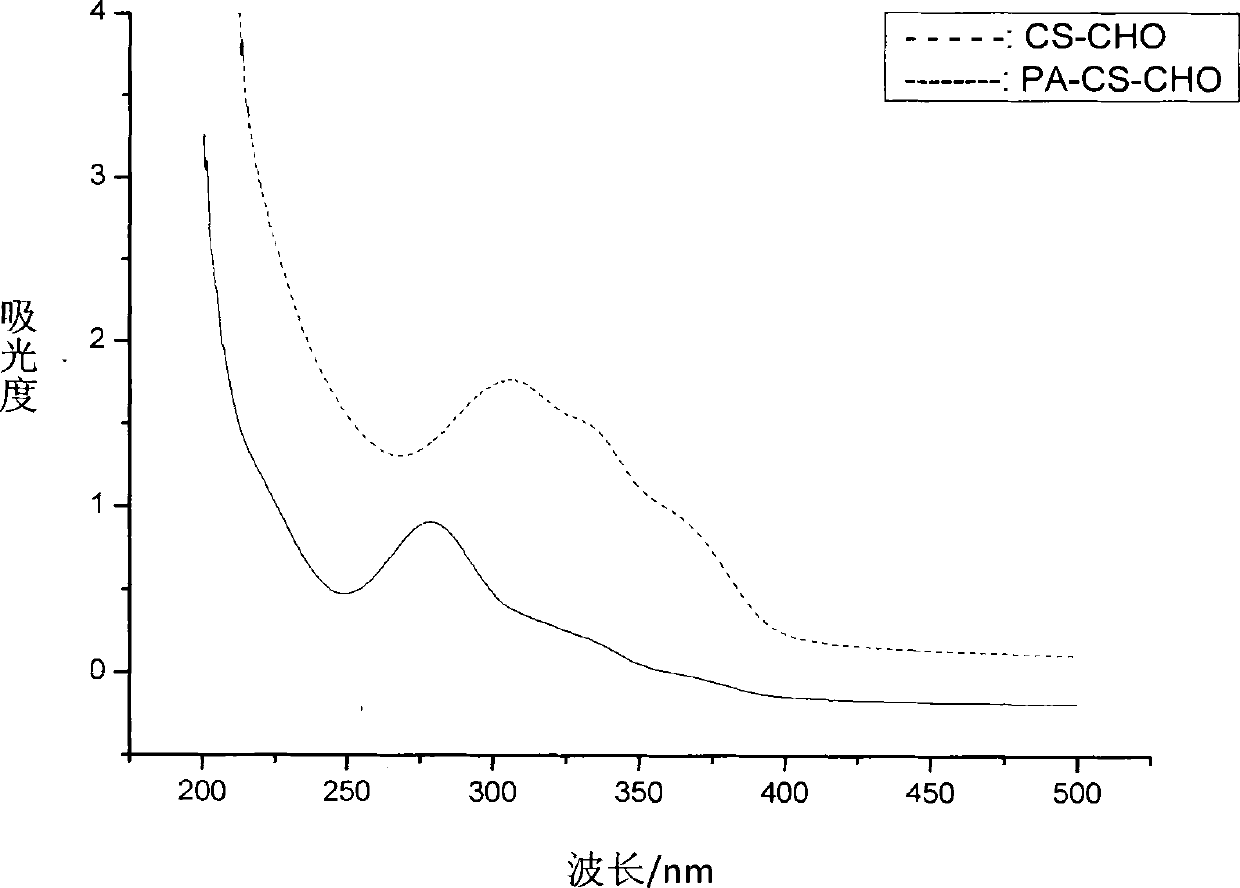
[0049] As a natural polysaccharide, chitosan has the characteristics of good biocompatibility and biodegradability, and is especially suitable for drug carriers. However, the main problem limiting its application is poor water solubility, which needs to be dissolved in acidic medium. Therefore, in this project, firstly, chitosan was oxidized with periodate to obtain chitosan with different molecular weights and chitosan chains containing o-dialdehyde functional groups, which greatly improved its water solubility. The synthetic route is as follows:
[0050]
[0051] Wherein, the range of x is 2-30; the range of y is 10-50, the range of y' is 2-20; the range of z is 8-30, wherein y=y'+z.
[0052] Under the protection of nitrogen, add N-hydroxysuccinimide to the aqueous solution of propiolic acid, dissolve it and drop it into the aqueous solution of aldo-chitosan CS-CHO, stir well, then add 1-ethyl-(3- Dimethylaminopro...
Embodiment 2
[0066] The synthesis of embodiment 2 azide probes
[0067]Palmitic acid with hexadecyl group can fix the "probe" in the phospholipid layer of LDL. In order to prevent the functional group - azide end group from being completely buried in LDL and lose its reactivity, we introduced a single azide The ethylene glycol unit is used as a "spacer" to synthesize N-(2-(2-azidoethoxy)ethyl)palmitamide (NAEP). Concrete synthetic route is as follows:
[0068]
[0069] Among them, a, 2-(2-aminoethoxy)ethanol, 1N HBTU and HOBt, reacted in DMF solution at room temperature for 24 hours; b, TsCl, pyridine; c, 1.2N sodium nitride, pyridine.
[0070] (1)C 15 h 31 CONHCH 2 CH 2 OCH 2 CH 2 Synthesis of OH(NHEP)
[0071] Dissolve 1 mol of palmitic acid, 1 mol of 2-(2-aminoethoxy)ethanol and 3 mol of triethylamine in a nitrogen atmosphere, add 3 mol of dimethylformamide (DMF) and stir to dissolve, place in an ice bath, cool and then add Mix 2 mol of benzotriazole-N, N, N', N'-tetramethyl...
Embodiment 3
[0082] Example 3 Probe labeling of LDL
[0083] LDL was extracted from human plasma by density gradient method (reference: Lundberg, B. Preparation of drug-low density lipoprotein complexes for delivery of antitumoral drugs via the low density lipoprotein pathway. Cancer Res. 1987, 47, 4105). NAEP and LDL are mixed in a co-solvent, and the LDL with an azide probe on the surface is obtained through a self-assembly reaction (NAEP-LDL, denoted as N 3 -LDL).
[0084] The particle size distribution and zeta potential between NAEP-LDL and native LDL after compounding are shown in Table 1 below:
[0085] Table 1 Composition, average particle size and zeta potential of pristine LDL and NAEP-LDL
[0086]
[0087] According to Table 1, it can be found that the particle size distribution and zeta potential difference between the compounded NAEP-LDL and the original LDL are not much different, indicating that the hydrophobic probe modification does not change much the mobility of LDL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com