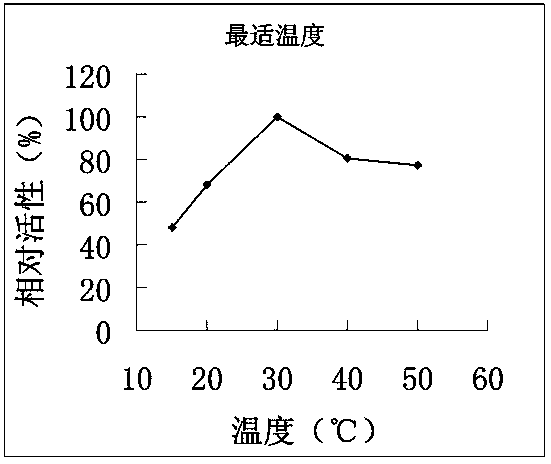
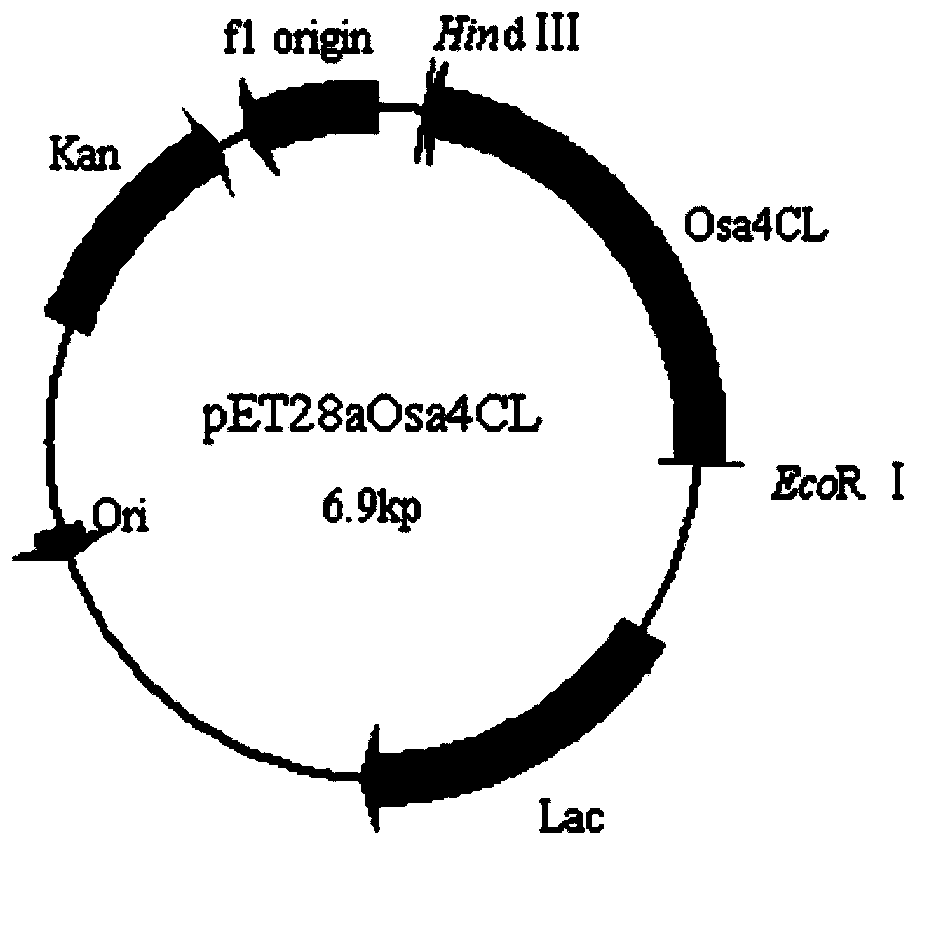
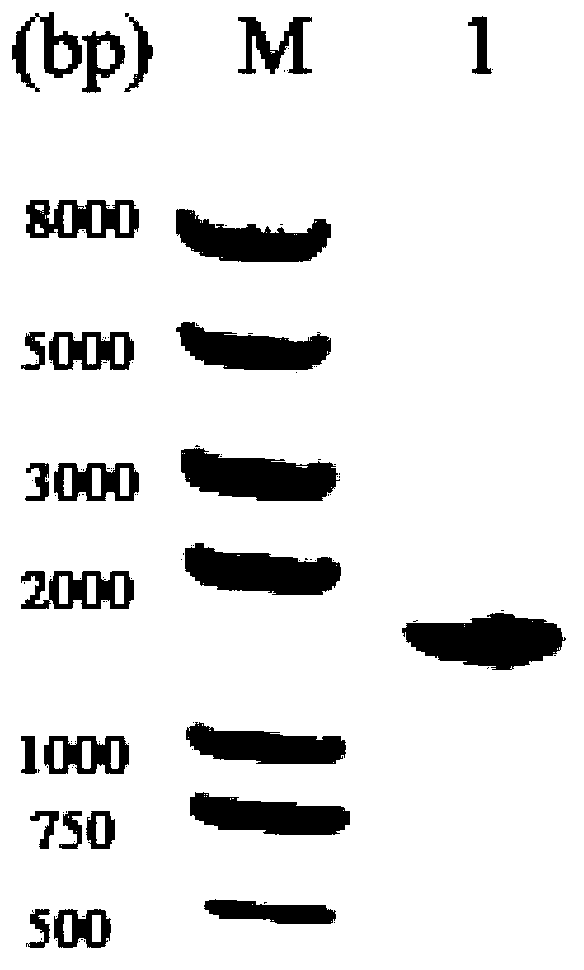4-coumarate coenzyme a ligase originated from ornithogalum caudatum, nucleotide sequence, and applications thereof
A technology of coumaric acid and ligase, applied in the field of genetic engineering, can solve problems such as low yield, environmental pollution of chemical synthesis, and shortage of resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1. Transcriptome Sequencing and Sequence Analysis
[0050] After extracting the total RNA from the sterile bulb of Dieffenbachia tiger eye, using the mRNA as a template, the first cDNA strand was synthesized with six base random primers (random hexamers), and then the second strand was synthesized by adding buffer, dNTPs, RNase H and DNA polymerase I After the cDNA chain is purified by QiaQuick PCR kit and eluted with EB buffer, the end is repaired, poly (A) is added, and the sequencing adapter is connected, and then the size of the fragment is selected by agarose gel electrophoresis, and finally PCR amplification is performed. The built sequencing library was used with Illumina HiSeq TM 2000 for sequencing.
[0051] The original image data obtained by sequencing is converted into sequence data through base calling, that is, raw data or raw reads. Perform data filtering on raw reads, remove reads with adapters, duplicates, and low-quality sequencing, and obtai...
Embodiment 2
[0053] Example 2 Osa4CL bioinformatics analysis
[0054] Using NCBI's ORF Finder (http: / / www.ncbi.nlm.nih.gov / gorf / orfig.cgi) software to analyze the ORF of SEQ ID NO.3, it was found that it contained a 1638bp 4- The coumaric acid-CoA ligase ORF is named Osa4CL, and its sequence is shown in SEQ ID NO.2. Using the tool ProtParam in Expasy (http: / / web.expasy.org / protparam / ) to predict the physicochemical properties of the protein encoded by Osa4CL, the results show that the Osa4CL protein encodes 545 amino acids with a molecular mass of 59.5332kDa and a theoretical isoelectric point of 5.37. Analysis by TMpred (http: / / www.ch.embnet.org / software / TMPRED_form.html) shows that the protein has two transmembrane domains, which are composed of 26 amino acids from 82 to 107. The domain from the inside to the outside of the membrane and the domain from the outside to the inside of the membrane composed of positions 232 to 257. Therefore, it is considered that the encoded protein is li...
Embodiment 3
[0057] Embodiment 3 Osa4CL gene cloning
[0058] Take 100mg of aseptic bulbs of Dieffenbachia tiger's eye, freeze them quickly in liquid nitrogen, grind them into a fine powder with a mortar, and extract total RNA from the callus of Dieffenbachia tiger's eye according to the instructions of RNeasy Plant Mini Kit (QIAGEN). RT-PCR Kit (ReverTra-Plus-, TOYOBO) was used to reverse transcribe the total RNA from the callus of Dieffenbachia tiger's eye into cDNA. The reverse transcription system and procedure are as follows: Add 1 μg of total RNA to 20 μL system, RNase Free H 2 O4 μL, Olig(dT) 20 1 μL, after incubating at 65°C for 5 minutes, immediately place it on ice to cool, then add 4 μL of 5×RT buffer, 1 μL of RNase Inhibitor (10U / μL), 1 μL of dNTP Mixture (10mM) and ReverTra Ace to the above tube, and incubate at 30°C for 10 minutes , incubated at 42°C for 60 minutes, denatured at 85°C for 5 minutes, and placed on ice for 5 minutes to complete cDNA synthesis. The cDNA was st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com