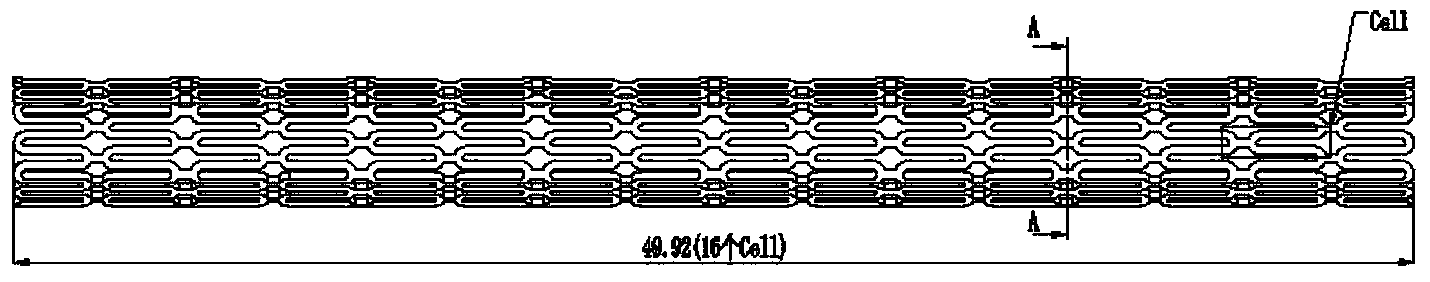
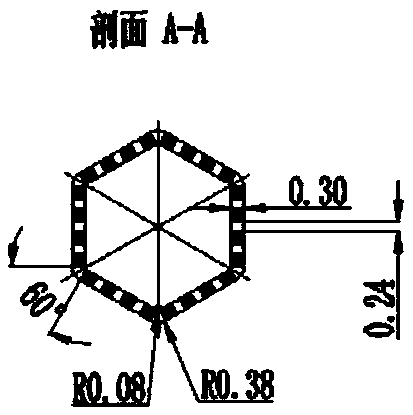
Method for preparing intravascular stent upon 3D (three-dimensional) printing technology
A 3D printing, 3D printer technology, applied in brackets and other directions, can solve problems such as hazards, microstructure defects, stepped surfaces, etc., to achieve the effects of cost reduction, good biocompatibility, and complete surface
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Step (1): Modeling
[0046] Establishing a 3D model of the intravascular stent in a computer, and decomposing the 3D model into a series of two-dimensional thin slice models with a thickness of 90 μm;
[0047] Step (2): Ingredients
[0048] Powder material: The stainless steel powder material is made by mixing spherical 316L stainless steel powder with an average particle size of 8 μm and stearic acid powder at a volume ratio of 60:40 at a temperature of 140°C;
[0049] Binder: a paraffin-based binder is made by mixing paraffin, low-density polyethylene, polypropylene and stearic acid in a mass ratio of 68:23:8:1;
[0050] Step (3): 3D printing to prepare green body
[0051] Input the model data built in step (1) into the supporting equipment of the 3D printer to set the printing program, send the stainless steel powder material in step (2) to the printing platform of the 3D printer, roll the layer, and the print head of the 3D printer The paraffin-based binder in th...
Embodiment 2
[0056] Step (1): Modeling
[0057] Establishing a 3D model of the intravascular stent in a computer, and decomposing the 3D model into a series of two-dimensional thin slice models with a thickness of 100 μm;
[0058] Step (2): Ingredients
[0059] Powder material: The stainless steel powder material is made by mixing spherical 316L stainless steel powder with an average particle size of 15 μm and stearic acid powder at a volume ratio of 80:20 at a temperature of 155°C;
[0060] Binder: a paraffin-based binder is made by mixing paraffin wax, low-density polyethylene, polypropylene and stearic acid in a mass ratio of 70:20:9:1;
[0061] Step (3): 3D printing to prepare green body
[0062] Input the model data built in step (1) into the supporting equipment of the 3D printer to set the printing program, send the stainless steel powder material in step (2) to the printing platform of the 3D printer, roll the layer, and the print head of the 3D printer The paraffin-based binder...
Embodiment 3
[0067] Step (1): Modeling
[0068] Establishing a 3D model of the intravascular stent in a computer, and decomposing the 3D model into a series of two-dimensional thin slice models with a thickness of 85 μm;
[0069] Step (2): Ingredients
[0070] Powder material: The nickel-titanium powder material is made by mixing spherical nickel-titanium powder with an average particle size of 10 μm and stearic acid powder at a volume ratio of 70:30 at a temperature of 145°C;
[0071] Adhesive: a cyano-adhesive with a mass percentage concentration of 0.8% made from α-ethyl cyanoacrylate dissolved in water;
[0072] Step (3): 3D printing to prepare green body
[0073] Input the model data built in step (1) into the supporting equipment of the 3D printer to set the printing program, send the nickel-titanium powder material in step (2) to the printing platform of the 3D printer, roll the layer, and print the 3D printer The cyano-based binder in the head spraying step (2) sticks the nickel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com