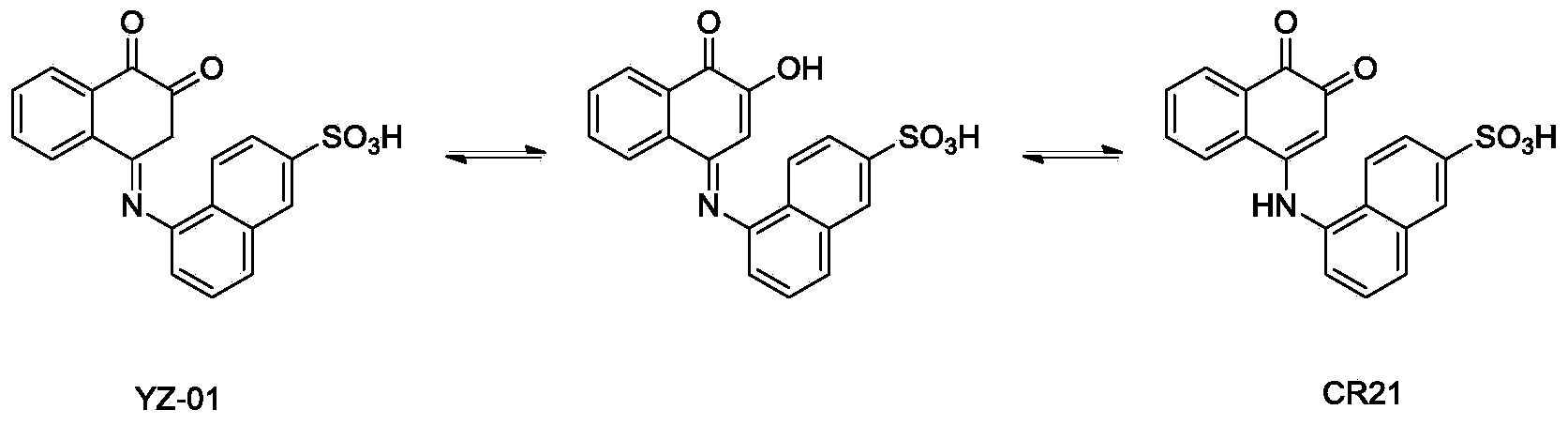
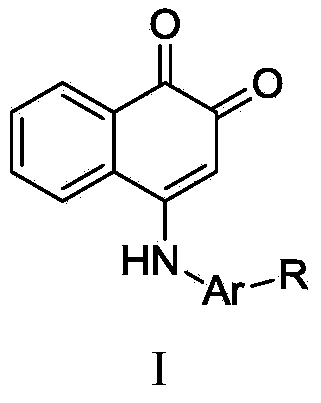
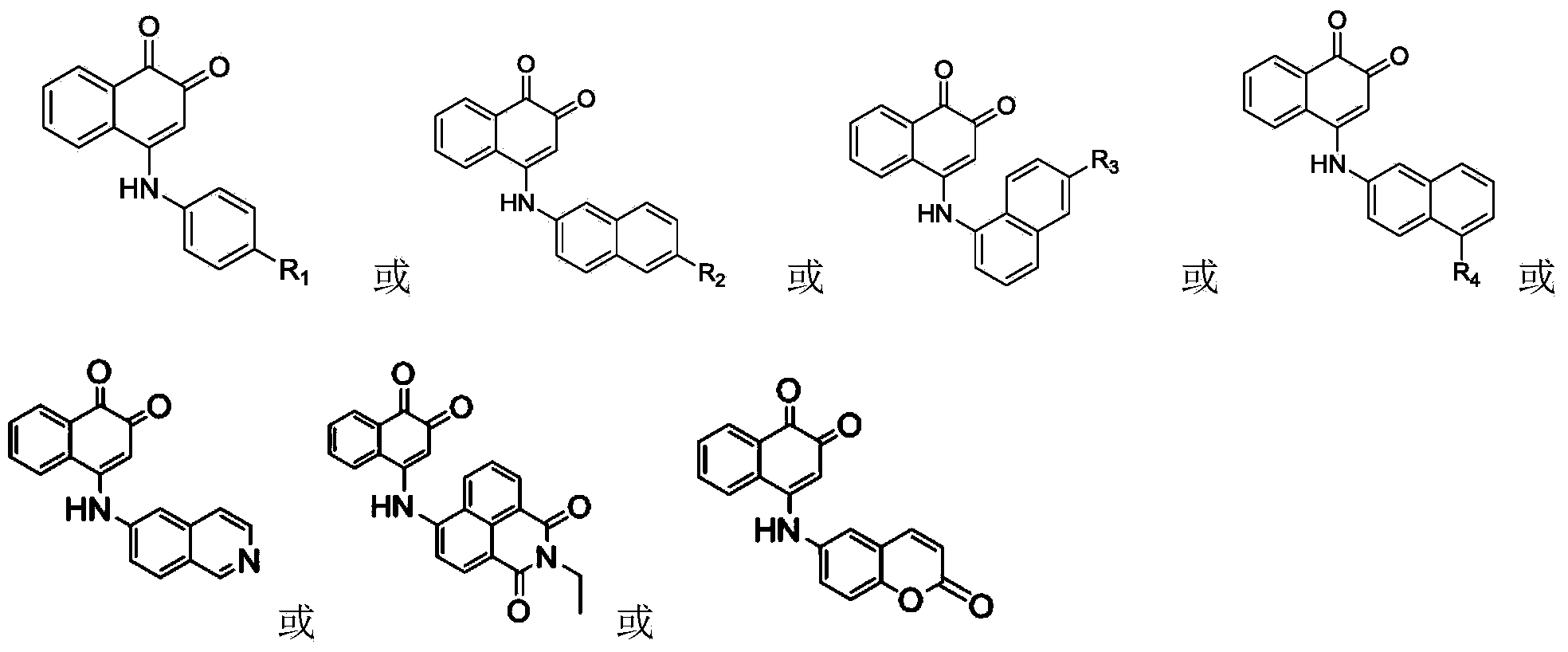
1,2-diketone naphthalene compound as well as preparation method and application thereof
A technology of diketone naphthalene and compounds, which is applied in the field of drug synthesis, can solve the problems of less research on CRAC channel blockers, blockade, low activity and selectivity of compounds, and achieve strong anti-inflammatory, mild reaction conditions, and easy-to-obtain raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1: Preparation of 4-((4-chlorophenyl)amino)-1,2-diketonenaphthalene (CR-3)
[0065] Sodium 1,2-naphthoquinone-4-sulfonate (52 mg, 0.2 mmol) and K 2 CO 3 (43 mg, 0.2 mmol) was dissolved in 50 mL of water, stirred until the solution was clear, and then p-chloroaniline (51 mg, 0.4 mmol) was dissolved in 5 mL of absolute ethanol, and then slowly added to the above aqueous solution, the solution rapidly changed to red and produces a red precipitate. After 1 h of reaction, filter, wash the filter residue with distilled water, and dry under vacuum at room temperature to obtain 46.6 mg of red powder with a reaction yield of 83.2%, m.p: 243-265°C. 1 H-NMR (300MHz, CDCl 3 ): δ=8.33(d, J=7.8Hz, 1H), 8.07(dd, J=7.5Hz, J=1.2Hz, 1H), 7.89(t, J=7.8Hz, 1H), 8.07(dt, J =7.8Hz, J=0.9Hz, 1H), 7.55(d, J=11.4Hz, 2H), 7.24(br.s, 2H), 5.94(br.s, 1H); ESI-HRMS: m / z calcd for C 16 h 10 ClNO 2 [(M+H) + ], 284.0478; found, 284.0546.
Embodiment 2
[0066] Example 2: Preparation of 4-((4-sulfonic acid phenyl)amino)-1,2-diketone naphthalene (CR-7)
[0067] React with 1,2-naphthoquinone-4-sodium sulfonate (52 mg, 0.2 mmol) and p-aminobenzenesulfonic acid (70 mg, 0.4 mmol) as raw materials, and the preparation method is the same as in Example 1 to obtain 45 mg of red powder , the yield was 68.1%. m.p.: 189-208°C. 1 H-NMR (300 MHz, CDCl 3 ):δ=11.67(brs,1H),8.02(dt,J=6.9Hz,J=0.9Hz,2H),7.96(d,J=1.5Hz,1H),7.94(t,J=1.2Hz,1H ),7.87~7.78(m,4H),6.18(s,1H);ESI-HRMS:m / z calcd for C 16 h 11 NO 5 S[(M-H) - ], 328.0280; found, 328.0318.
Embodiment 3
[0068] Embodiment 3: Preparation of 4-((naphthalene-2-amino)-1,2-diketonenaphthalene (CR-8)
[0069] 1,2-naphthoquinone-4-sodium sulfonate (52 mg, 0.2 mmol) and 2-naphthylamine (58 mg, 0.4 mmol) were used as raw materials for the reaction, and the preparation method was the same as in Example 1 to obtain 48.2 mg of red powder, Yield 82.6%, m.p: 285-292°C. 1 H-NMR (300MHz, CDCl 3 ):δ=8.41(d,J=7.8Hz,1H),8.10~8.06(m,3H),8.00~7.93(m,2H),7.90~7.88(m,1H),7.80~7.75(m,1H ),7.59~7.53(m,2H),7.52(br.s,1H),6.20(br.s,1H);ESI-HRMS:m / z calcd for C 20 h 13 NO 2 [(M+H) + ], 300.1025; found, 300.0989.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com