Preparation and application of macrolide compound
A technology of macrolide and compound is applied in the field of preparation of macrolide compound to achieve the effect of low toxicity agricultural insecticidal and acaricidal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example
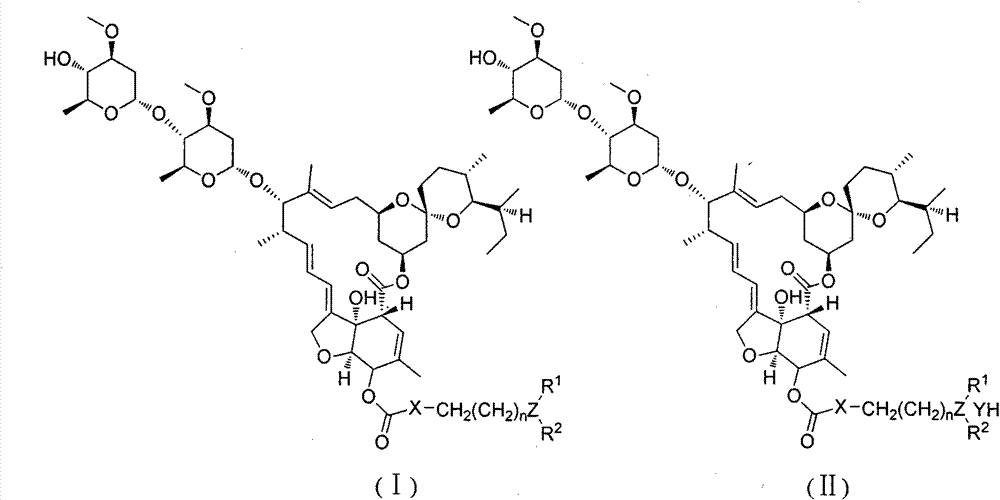
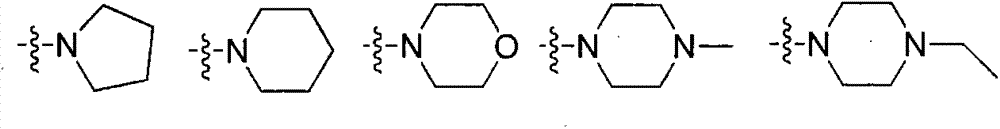
[0031] Preparation of intermediate 15-O-p-nitrophenyl carbonate ivermectin B1a
[0032] Add dry ivermectin B1a15.63g (18mmol) to a 250ml round bottom flask, dissolve in 150ml tetrahydrofuran, and add 2.09g (18mmol) tetramethylethylenediamine dropwise in an ice-salt bath. After stirring for 10 minutes, add 7.21g (36mmol) p-nitrophenyl chloroformate, and after the system turns into a brown turbid system, remove the ice-salt bath and change to stirring at room temperature. TLC followed the reaction until the raw material point did not change and then stopped stirring. The insoluble matter was removed by suction filtration, and the filtrate was rotary evaporated to remove tetrahydrofuran to obtain a yellow solid. 150ml of dichloromethane was added to the obtained solid to obtain a brown turbid system, which was washed with water (60ml×3) and then washed once with saturated brine. After drying the organic phase with anhydrous sodium sulfate, the solvent was removed to obtain a cr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com