Solid phase coating method for hyaluronic acid
A technology of hyaluronic acid and solid-phase carrier, applied in the field of immunoassay medicine, can solve the problems of unstable composition, affecting the accuracy and precision of test results, etc., to enhance stability, improve precision and reliability, and shorten the The effect of reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 1. Treatment of hyaluronic acid:
[0021] (1) Weigh 5mg hyaluronic acid and dissolve it in 1ml distilled water;
[0022] (2) Add 0.1ml of 1.5mol / L hydrochloric acid and react overnight at 2-8°C;
[0023] (3) Add 0.1ml1.5mol / L NaOH to neutralize to neutral;
[0024] (4) Borate buffer dialysis for 2 hours to remove excess salt ions;
[0025] 2. Reaction of NHS-activated biotin and treated hyaluronic acid:
[0026] (1) Dissolve 0.5 mg of NHS-LC-LC-Biotin in 0.5 ml of distilled water; add the Biotin solution into the hydrolyzed hyaluronic acid solution, and shake it in the dark for 4 hours to obtain the HA-Biotin complex and unbound NHS -LC-LC-Biotin mixed solution;
[0027] (2) Weigh 53.5mg NH 4 Dissolve Cl in 1ml of distilled water to prepare 1mol / LNH 4 Cl solution;
[0028] (3) Add the solution prepared in (2) to (1), and shake it for 4 hours at 2-8°C under dark conditions;
[0029] (4) Dialyze with phosphate buffer at 2-8° C. for 24 hours to obtain HA-biotin com...
Embodiment 2
[0038] Embodiment 2: Coated plate accelerated stability test
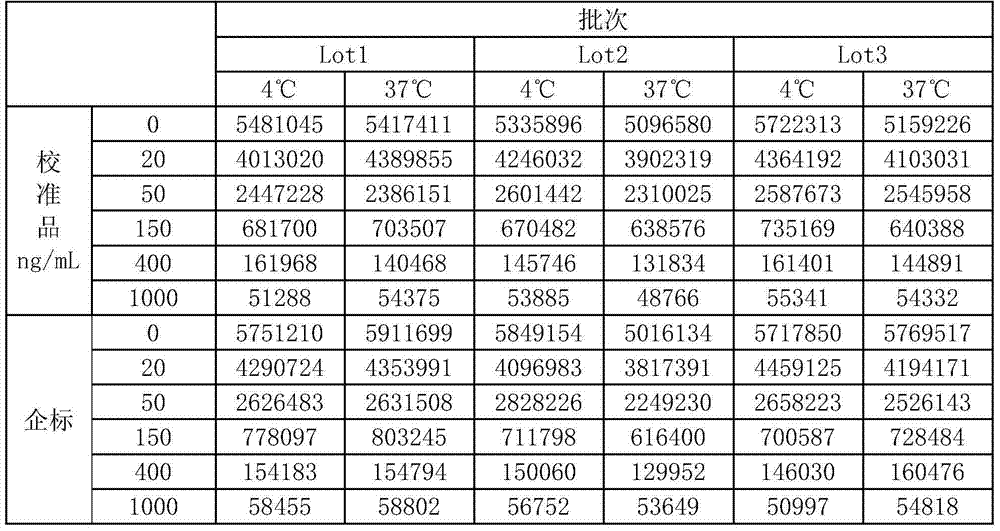
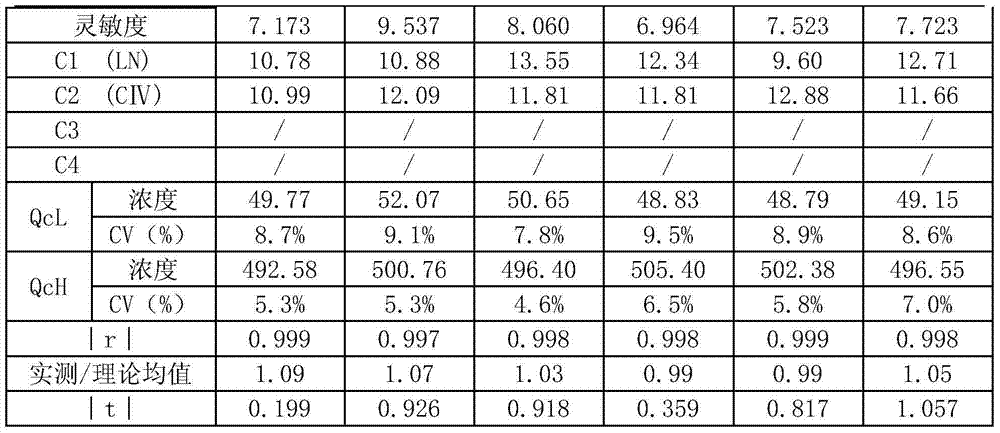
[0039] Use the method of the present invention to coat a 96-well luminescent plate, and coat three batches. Each batch is divided into two parts, one part is placed in a 4°C refrigerator, and the other part is placed in a 37°C refrigerator for accelerated stability. Take it out after 7 days, equilibrate to room temperature, and measure Calibrator, enterprise standard, quality control product, test the precision, sensitivity, and linearity of the coated plate to judge its stability. The experimental results are as follows:
[0040]
[0041]
[0042] It can be seen from the table that when the coated plate is placed at 37°C for 7 days, compared with 4°C, there is no significant difference in the sensitivity, precision and linearity of quality control products, and the stability is very good, and the precision is not affected .
Embodiment 3
[0043] Embodiment 3: Accuracy test of measured value of coated plate
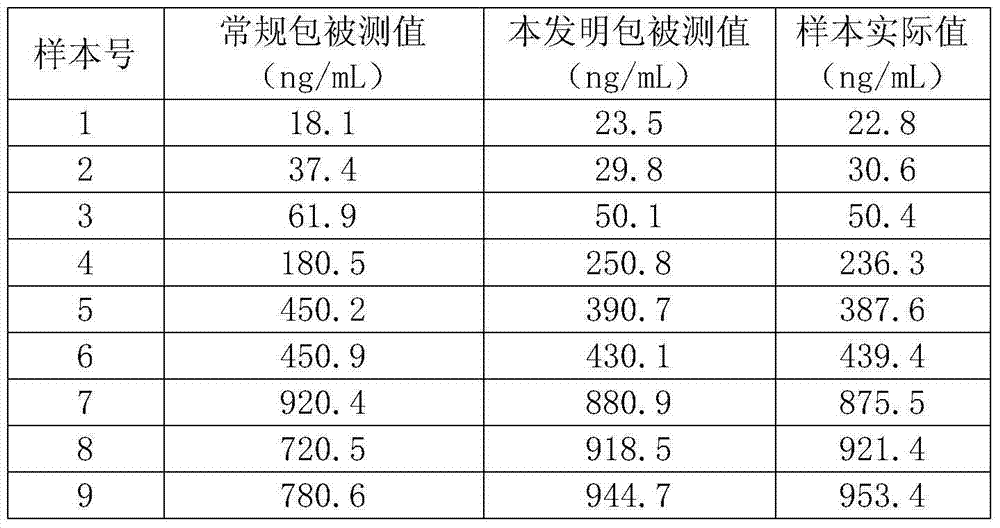
[0044] Adopt conventional method and the method of the present invention to coat hyaluronic acid respectively, measure 9 samples, high value, medium value, low value respectively 3, compare the accuracy of the measured value of two groups of packs, obtain the following results:
[0045]
[0046] As can be seen from the table, the measurement accuracy of the coating method of the present invention is significantly improved compared with conventional methods.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com