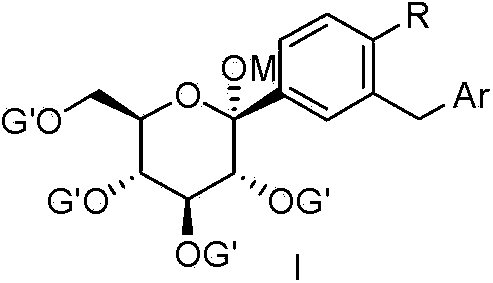
Method for preparing Invokana medicine intermediate by using micro-reactor
A micro-reactor and micro-reaction technology, which is applied in chemical instruments and methods, chemical/physical/physical chemical reactors, preparation of sugar derivatives, etc., to achieve high safety performance, shorten reaction time, and improve reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
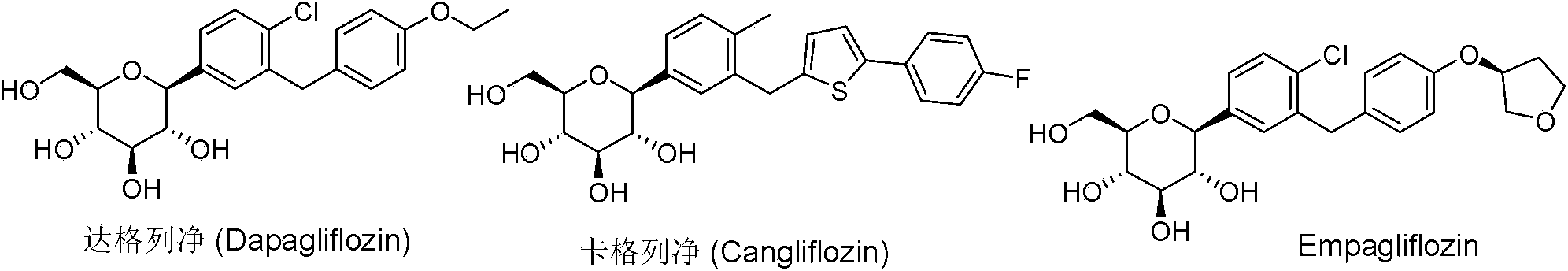
[0027] Example 1 Dapagliflozin Intermediate Ia--(3R,4S,5S,6R)-2-(4-chloro-3-(4-ethoxybenzyl)phenyl)-6-(hydroxymethyl) - Synthesis of 2-methoxytetrahydro-2H-pyran-3,4,5-triol.
[0028]
[0029] method 1
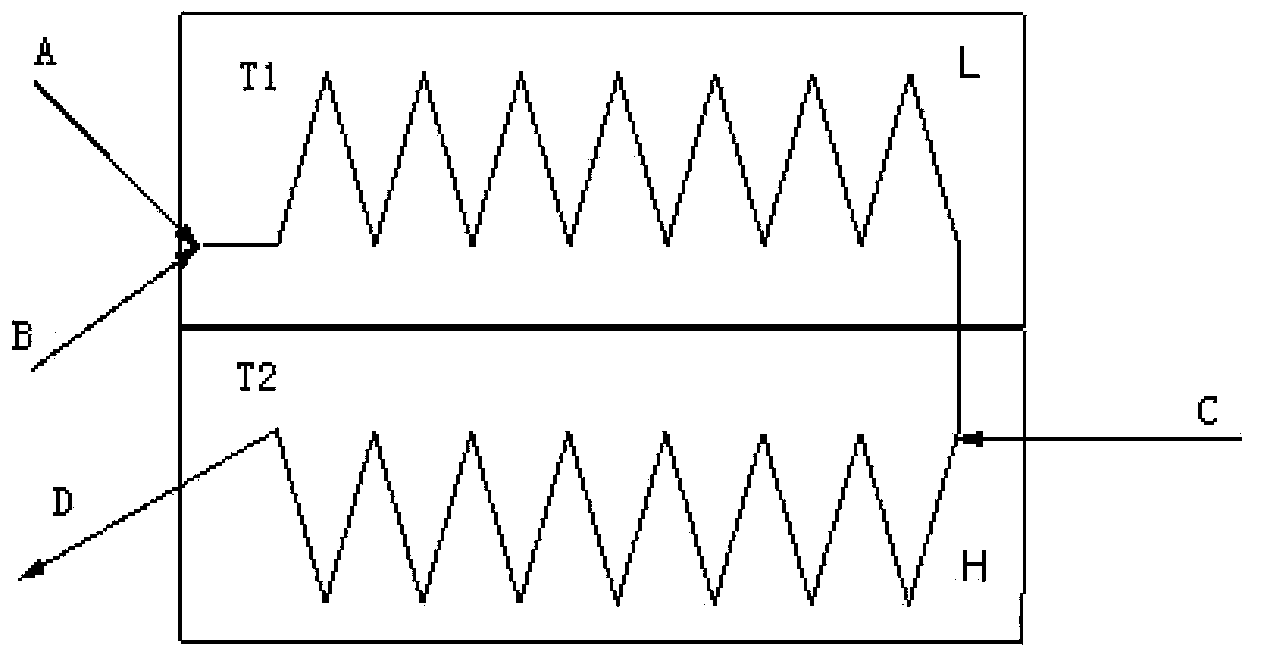
[0030] Prepare 163mL (163g, 0.5mol) of the ether solution of 4-bromo-1-chloro-2-(4-ethoxybenzyl)benzene of 1g / mL, feed the above ether solution into material channel A, and control the flow rate to be 0.1 mL / min. At the same time, a total of 200 mL of 2.5M n-butyllithium solution was passed into the material channel B, and the flow rate was controlled to be 0.12 mL / min, wherein the preset temperature T1 of the micro-reaction unit L was -80~-75°C. Then, 0.5 g / mL of (3R,4S,5R,6R)-3,4,5-tris(trimethylsiloxy)-6-((trimethylsiloxy) was passed into material channel C The ether solution of methyl) tetrahydro-2H-pyran-2-one 514mL (257g, 0.55mol), the control flow rate is 0.3mL / min, wherein the preset temperature T2 of the micro reaction unit H is -80~-70 ℃ . After the completio...
example 2
[0035] Example 2 Canagliflozin Intermediate Ib--(3R,4S,5R,6R)-6-(acetylmethyl)-2-(3-((5-(4-fluorophenyl)thiophen-2-yl) )Methyl)-4-methylphenyl)-2-tetrahydro-2H-pyran-3,4,5-triacetate synthesis.
[0036]
[0037] Prepare 2040mL (204g, 0.5mol) of a toluene solution of 2-(2-methyl-5-iodobenzyl)-5-(4-fluorophenyl)thiophene at 0.1g / mL, and feed the above into material channel A Toluene solution, control flow rate 2mL / min. At the same time, a total of 250 mL of 2M isopropyl Grignard reagent and lithium chloride solution (the molar ratio of Grignard reagent and lithium chloride is 1:1) was passed into the material channel B, and the control flow rate was 0.23 mL / min. The preset temperature T1 of L is -10~0℃. Then, 0.2 g / mL of (2R,3R,4S,5R)-2-(acetoxymethyl)-6-carbonyltetrahydro-2H-pyran-3,4,5-tris was passed into material channel C The tetrahydrofuran solution of acetate was 952 mL (190 g, 0.55 mol), and the flow rate was controlled to be 0.9 mL / min, and the preset temperature ...
example 3
[0039] Example 3 Canagliflozin Intermediate Ic--(3R,4S,5R,6R)-3,4,5-tris(benzyloxy)-6-(benzyloxymethyl)-2-(3-(( Synthesis of 5-(4-fluorophenyl)thiophen-2-yl)methyl)-4-methylphenyl)tetrahydro-2H-pyran-2-oxane.
[0040]
[0041] Prepare 2040mL (204g, 0.5mol) of a toluene solution of 2-(2-methyl-5-iodobenzyl)-5-(4-fluorophenyl)thiophene at 0.1g / mL, and feed the above into material channel A Toluene solution, control speed 2mL / min. At the same time, a total of 250 mL of 2M ethyl Grignard reagent and lithium chloride solution (the molar ratio of Grignard reagent to lithium chloride is 1:1.2) was passed into channel B, and the flow rate was controlled to 0.23 mL / min. The temperature T1 is -10 to 0°C. Then 0.2 g / mL of (3R,4S,5R,6R)-3,4,5-tris(benzyloxy)-6-(benzyloxymethyl)tetrahydro-2H-pyridine was passed into feed channel C 1480 mL (296 g, 0.55 mol) of the tetrahydrofuran solution of furan-2-one, the flow rate was controlled to be 1.4 mL / min, and the preset temperature T2 of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com