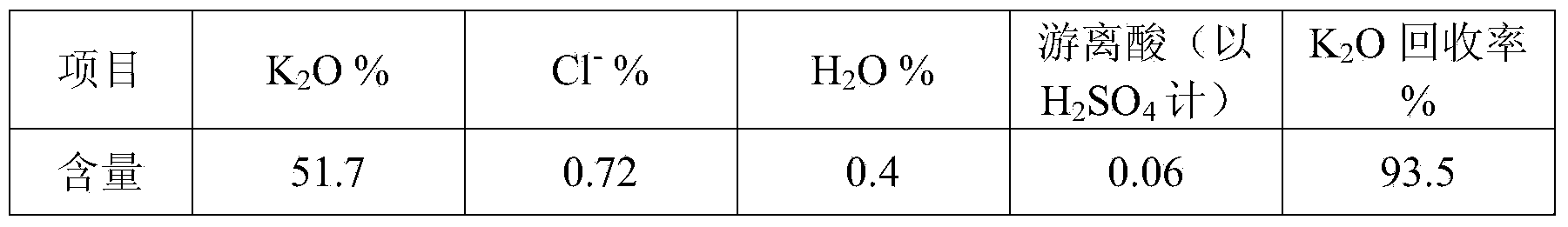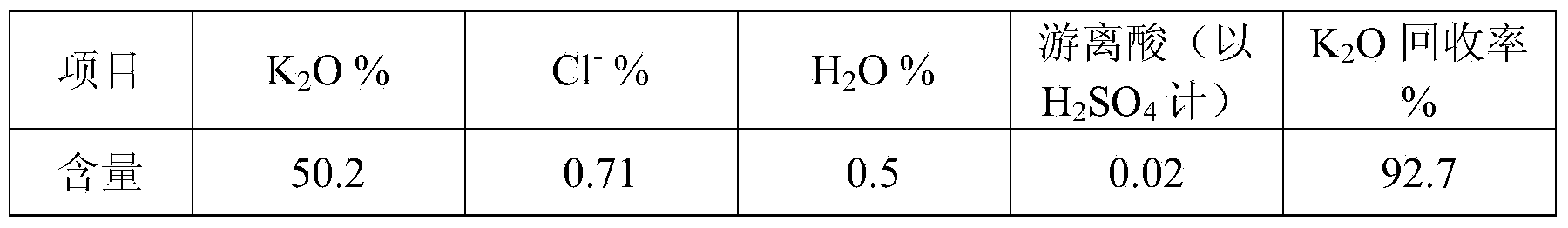Method for producing potassium sulfate from potassium/chlorine-ion-containing solid waste
A chlorine ion and solid waste technology, applied in the direction of sulfate/bisulfate preparation, etc., can solve the problems of unreported waste water utilization technology, failure to be popularized and applied, complicated process, etc., and achieve low production cost, small investment, Good effect on operational safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
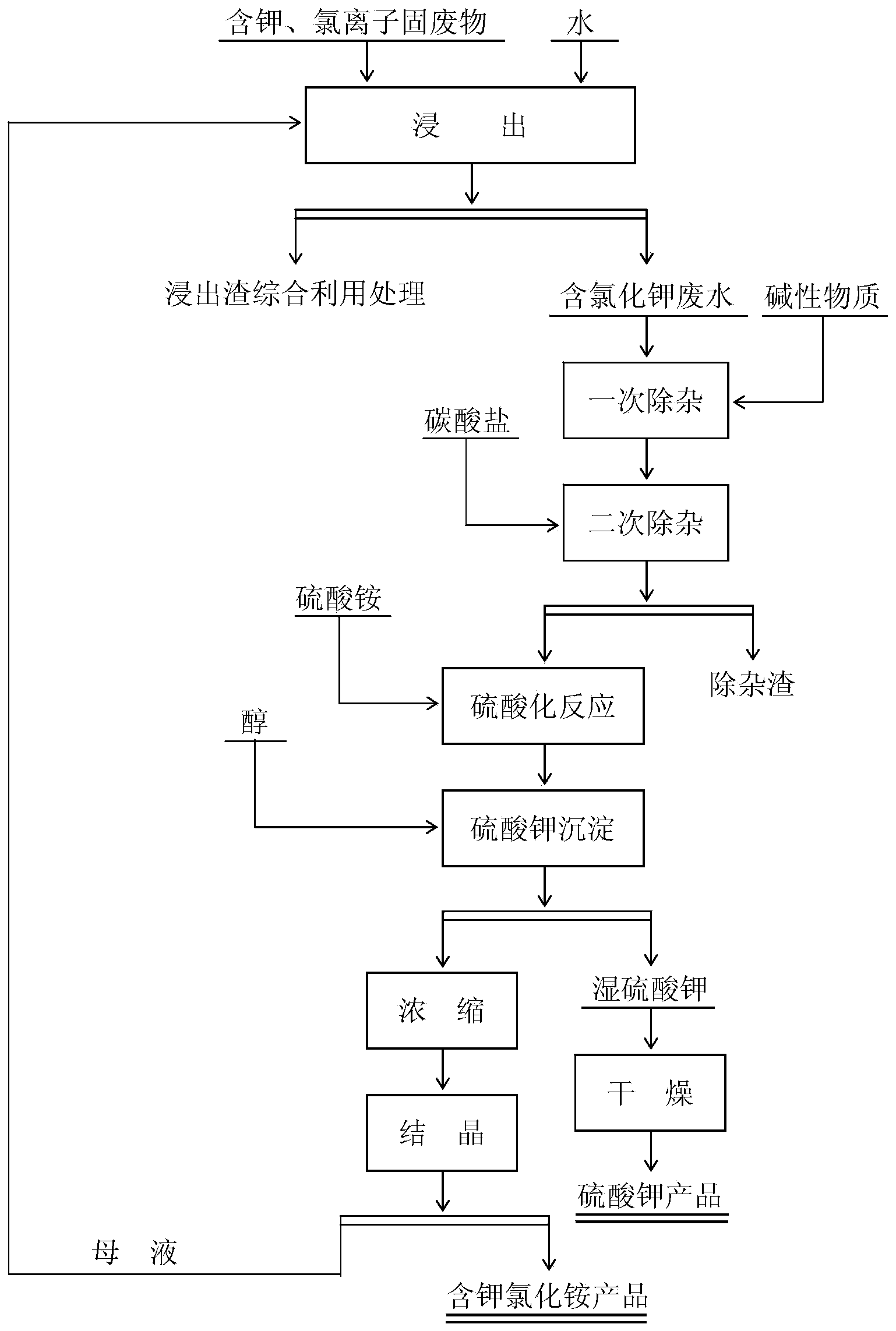
[0043] 1. Remove impurities
[0044] To the leaching wastewater containing potassium and chloride ion solid waste (main component: K 2 O content is 4.7%, Cl ion content is 5.8%), add lime (add-on is 0.08% of solution weight) and regulate the pH value=11.5 of solution, after stirring and removing impurity 60min, add ammonium carbonate (add-on) in solution again 0.08% of the solution weight), after stirring for 60 minutes to remove impurities, filter, and the filtrate enters the buffer tank for subsequent use.
[0045] 2. Sulfation reaction
[0046] Add the solution in the buffer tank to the reaction tank, and simultaneously add 5.2% ammonium sulfate by solution weight (the molar ratio of ammonium sulfate to potassium chloride=1.85). After stirring for 180 min, the solution enters the potassium sulfate precipitation process.
[0047] 3. Potassium sulfate precipitation
[0048] The solution after the sulfation reaction is completed is added to the potassium sulfate precipitati...
Embodiment 2
[0058] 1. Remove impurities
[0059] To the leaching wastewater containing potassium and chloride ion solid waste (main component: K 2 O content is 4.9%, Cl ion content is 6.0%), add potassium hydroxide (add-on is 0.1% of solution weight) and regulate the pH value=13.5 of solution, after stirring and removing impurities 90min, add salt of wormwood in solution again ( The addition amount is 0.15% of the solution weight), after stirring for 70 minutes to remove impurities, filter, and the filtrate enters the buffer tank for standby.
[0060] 2. Sulfation reaction
[0061] Add the solution in the buffer tank to the reaction tank, and add 5.8% ammonium sulfate (the molar ratio of ammonium sulfate to potassium chloride=2.05) at the same time. After stirring for 150 minutes, the solution enters the potassium sulfate precipitation process.
[0062] 3. Potassium sulfate precipitation
[0063] Add the solution after the sulfation reaction to the potassium sulfate precipitation tank,...
Embodiment 3
[0073] 1. Remove impurities
[0074] To the leaching wastewater containing potassium and chloride ion solid waste (main component: K 2 O content is 9.2%, Cl ion content is 11.6%), add sodium carbonate (add-on is 1.5% of solution weight) and regulate the pH value=14 of solution, after stirring and removing impurity 90min, add ammonium carbonate (addition) in solution again The amount is 0.08% of the solution weight), after stirring for 90 minutes to remove impurities, filter, and the filtrate enters the buffer tank for subsequent use.
[0075] 2. Sulfation reaction
[0076] Add the solution in the buffer tank to the reaction tank, and add 11.7% ammonium sulfate (the molar ratio of ammonium sulfate to potassium chloride=2.5) at the same time. After stirring for 240 minutes, the solution enters the potassium sulfate precipitation process.
[0077] 3. Potassium sulfate precipitation
[0078] The solution after the sulfation reaction is completed is added to the potassium sulfat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com