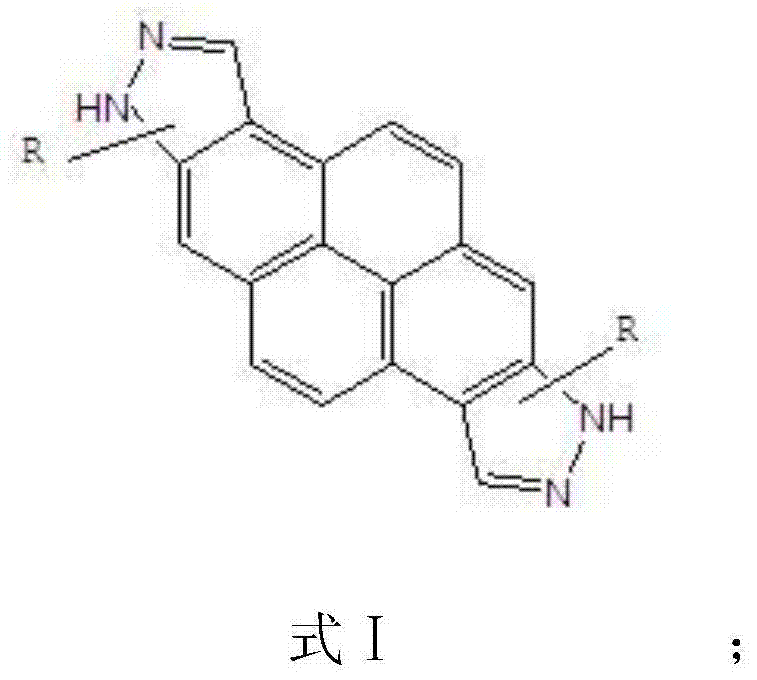
Blue light semi-conductor material containing diindazolepyrene and preparation method as well as organic light emitting apparatus made from material
A diindazole and semiconductor technology, applied in the field of organic light-emitting devices, can solve the problems of device spectral instability, and achieve the effects of good electron transport performance, high stability and improved mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: the synthesis of compound 1
[0038]
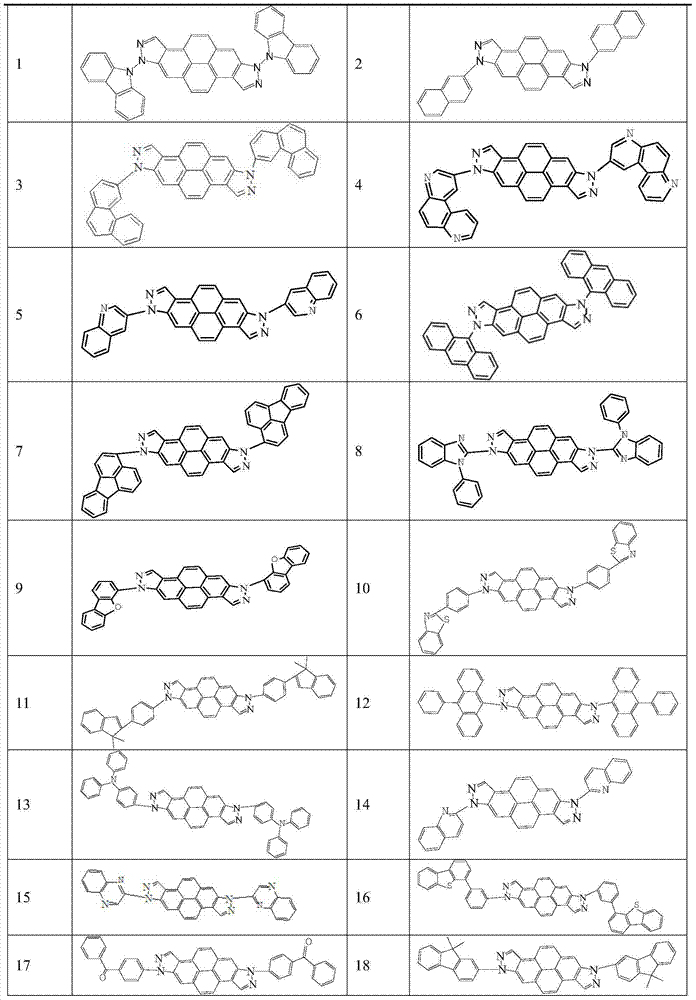
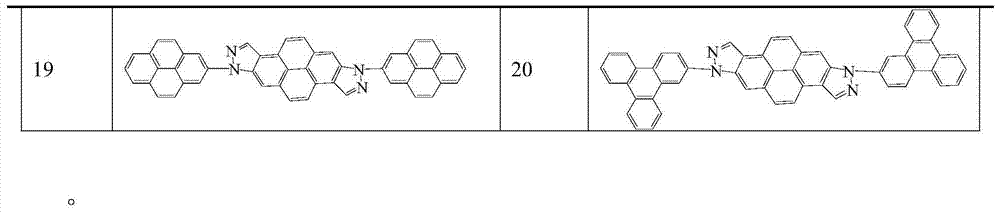
[0039] Under nitrogen protection, put 100mmol of bisindazolopyrene intermediate, 220mmol of 9-bromo-9H-carbazole, 5mmol of tris(dibenzylideneacetone)dipalladium, 400mmol of sodium tert-butoxide, and 20mmol of tri-tert-butylphosphine into a reaction vessel, and dissolved with 500ml of toluene, 110 ° C reflux reaction for 24h, with thin layer chromatography (TLC) to determine the end point of the reaction, the reaction is complete, cooled to room temperature, through a silica gel funnel, rinsed with dichloromethane, spin-dried, dichloromethane / Petroleum ether was recrystallized, filtered with suction, and dried to obtain 88 mmol of compound 1 with a yield of 88%.
Embodiment 2
[0040] Embodiment 2: the synthesis of compound 2
[0041]
[0042] Under nitrogen protection, put 100mmol of bisindazolopyrene intermediate, 215mmol of 2-bromonaphthalene, tris(dibenzylideneacetone) dipalladium (5mmol), 409mmol of sodium tert-butoxide, and 20mmol of tri-tert-butylphosphine into the reaction In the container, dissolve it with 500ml toluene, reflux at 110°C for 24h, determine the end point of the reaction with thin layer chromatography (TLC), after the reaction is complete, cool to room temperature, pass through a silica gel funnel, rinse with dichloromethane, spin dry, dichloromethane / petroleum ether After recrystallization, suction filtration and drying, 79 mmol of compound 2 was obtained with a yield of 79%.
Embodiment 3
[0043] Embodiment 3: the synthesis of compound 3
[0044]
[0045]Under nitrogen protection, put 102mmol of bisindazolopyrene intermediate, 219mmol of 3-bromophenanthrene, 5mmol of tris(dibenzylideneacetone)dipalladium, 404mmol of sodium tert-butoxide, and 20mmol of tri-tert-butylphosphine into a reaction vessel , and dissolved in 500ml of toluene, reflux at 110°C for 24h, use thin-layer chromatography (TLC) to determine the end point of the reaction, after the reaction is complete, cool to room temperature, pass through a silica gel funnel, rinse with dichloromethane, spin dry, dichloromethane / petroleum ether recrystallization , filtered by suction, and dried to obtain 74mmol of compound 3, with a yield of 73%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com