Erlotinib hydrochloride pharmaceutical composition without containing surfactant
A technology of erlotinib hydrochloride and surfactant, applied in the field of erlotinib hydrochloride pharmaceutical composition and preparation thereof, can solve the problems of easily destroying the stability of medicinal crystal forms, affecting the stability of products, etc., so as to improve safety sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
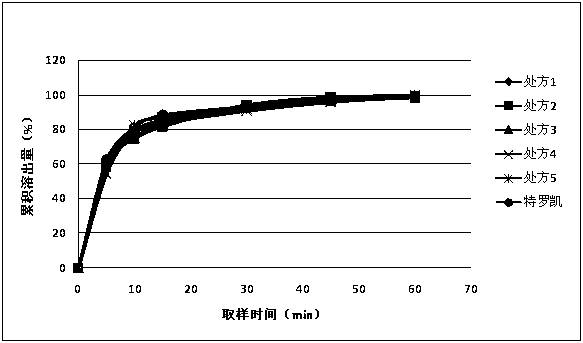
[0055] Weigh each component according to prescription 1, mix mannitol, lactose monohydrate and erlotinib hydrochloride evenly and pass through a 60-mesh sieve for micronization. The particle size distribution of the mixed material is required to be D10=0.1~5μm, D50=1~15μm , D90<30 μm. Take the mixture that meets the particle size distribution requirements to monitor the content of erlotinib hydrochloride, and convert the ratio in the material; take the mixture with known solid content, put it in the mixer, add microcrystalline cellulose PH101, sodium starch glycolate and mix for 10 minutes; collect material. Take the mixed material and put it on the dry granulator, adjust the rotation speed of the extrusion wheel (3.6~5.3rpm), the rotation speed of the feeding screw (12~24rpm), the pressure of the oil cylinder (the pressure of the oil cylinder is 1.0 MPa~3.0 MPa) to make it three Those who cooperate effectively, until the hardness of the pressed medicine block (1~3kg) i...
Embodiment 2~5
[0057] Each component is weighed according to prescription 2-5, and the preparation method is the same as in Example 1.
Embodiment 6
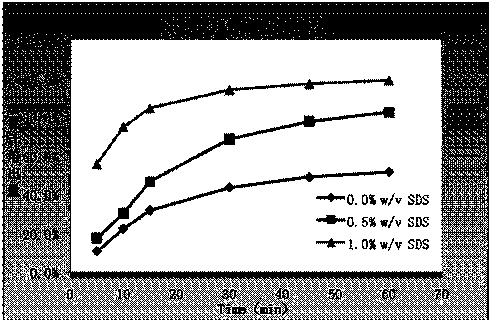
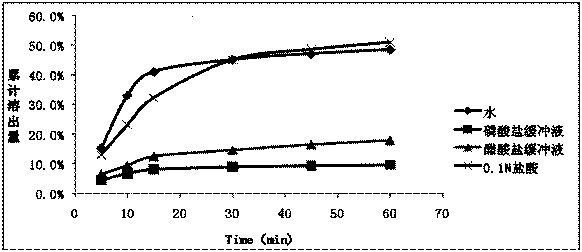
[0059] The dissolution rate measurement of embodiment 1~5 preparation
[0060] Dissolution method: paddle method, 75 rpm, solvent: 0.1mol / L hydrochloric acid solution (1%V / V~SDS) 1000ml.
[0061] At 5min, 10min, 15min, 30min, 45min, and 60min, take 10ml and filter it, dilute it with dissolution medium and measure it; the concentration of the reference solution is 10μg / ml. The drug concentration detection method is UV method; the detection wavelength is 352nm. The dissolution data are shown in Table 2.
[0062] Table 2
[0063] .
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com