An avian influenza vaccine composition, a preparing method thereof and applications of the composition
A vaccine composition and bird flu technology, applied in biochemical equipment and methods, vaccines, veterinary vaccines, etc., can solve problems such as high production costs, waste of manpower and material resources, and long vaccine production cycle, and achieve enhanced delivery , increase the penetrating ability, increase the effect of expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1 artificial synthesis M2, 3M2e protein
[0046] According to NCBI ( http: / / www.ncbi.nlm.nih.gov ) nucleotide sequences of M2 and M2e protein genes reported in A / Duck / Hong Kong / Y439 / 97 (H9N2, accession number: AF156462) (see sequence table SEQ ID No.1 and SEQ ID No.3 for details), The amino acid polypeptide sequences of M2 and 3M2e proteins were artificially synthesized (see sequence table SEQ ID No.2 and SEQ ID No.4 for details), and the synthesized polypeptide sequences were purified by high performance liquid chromatography to obtain purified M2 and 3M2e proteins respectively . Then, the M2 and 3M2e proteins were respectively prepared into 1 mg / mL stock solution with physiological saline containing 1% trehalose.
Embodiment 2
[0047] The preparation of embodiment 2 influenza vaccines
[0048] The film method of the preparation method of influenza vaccine of the present invention combines with freeze-drying method, comprises the following operation steps:
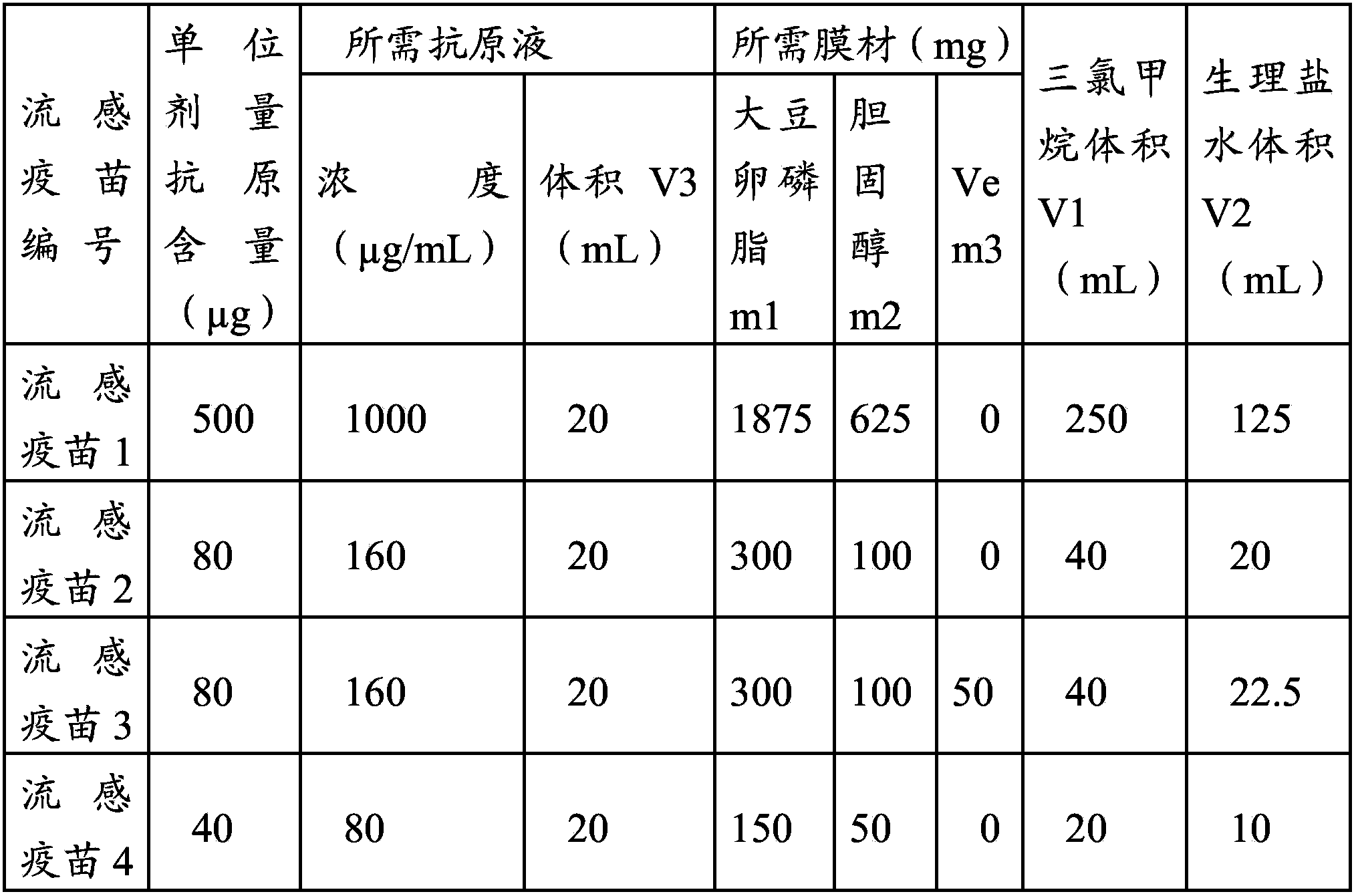
[0049] (1) Soybean lecithin m1, cholesterol m2, and vitamin E (Ve) m3 were mixed and dissolved in V1 chloroform according to the amount in Table 1, and the solution was placed in an eggplant-shaped bottle of a rotary evaporator, and a water bath at 45°C was used as Evaporation temperature, vacuum rotary evaporation, film formation.
[0050] (2) Add physiological saline V2 according to the final concentration of the film material at 2% (W / W), add a few small glass beads, and rotate to evaporate at 45°C. After the film completely falls off the bottle wall, let it stand for about 25 minutes to make the bottle Medium liquid is milky white suspension.
[0051] (3) Add different concentrations and volumes of avian influenza antigen solution (containin...
Embodiment 3
[0056] Embodiment 3 animal experiments
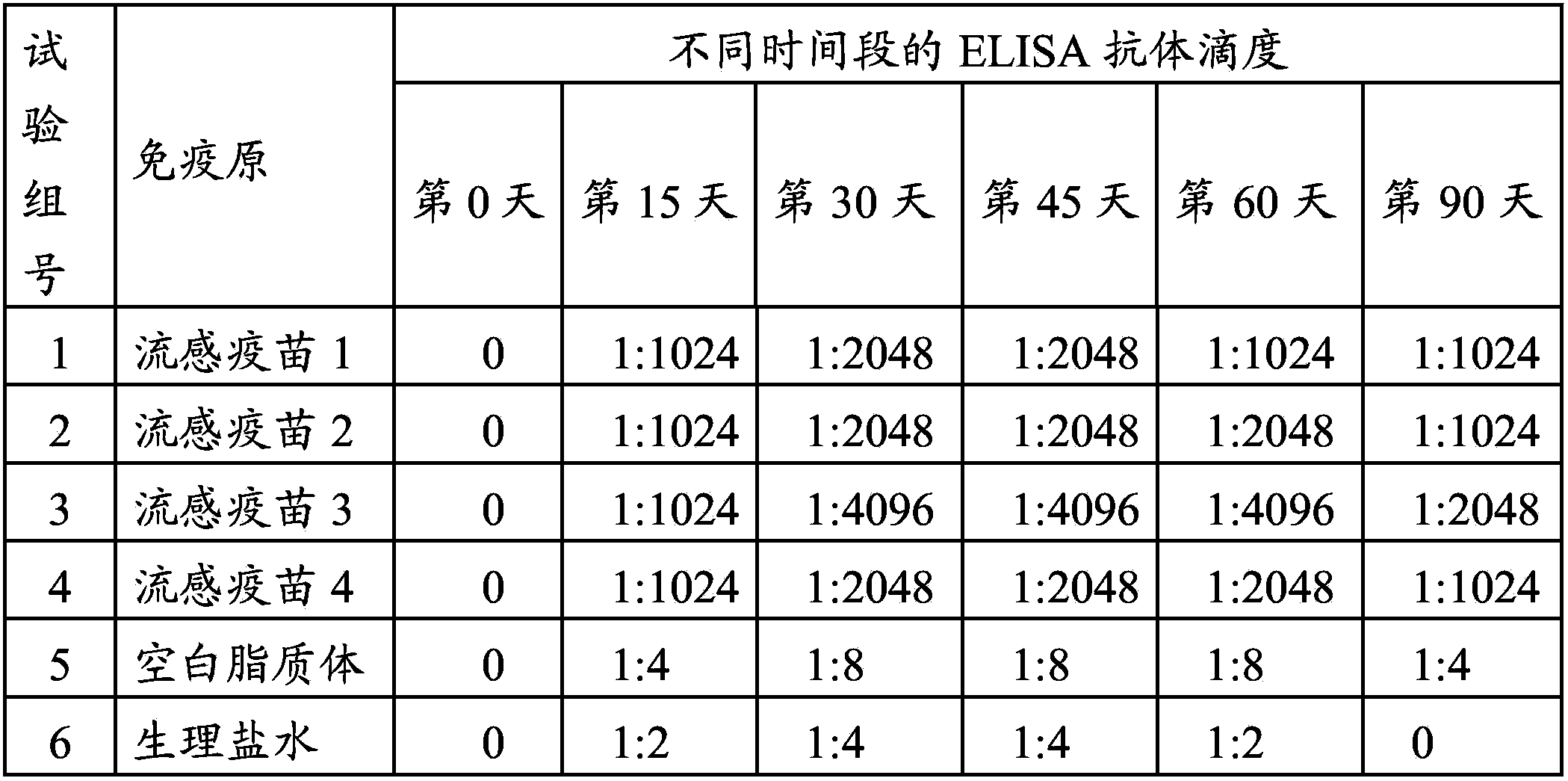
[0057] A total of 120 3-week-old SPF chickens were selected and randomly divided into 6 groups, 20 birds per group. They were subcutaneously injected with homemade influenza vaccine, blank liposomes, and normal saline (as a blank control) at a dose of 0.3 mL / feather.
[0058] On the 0th day, 15th day, 30th day, 45th day, 60th day, and 90th day after immunization, the serum of each test group was collected and separated. At the same time, the serum anti-M2 or M2e antibody titer was detected by indirect ELISA method. The specific operation steps were as follows: Dilute the M2 or M2e protein stock solution to 1 μg / mL with normal saline, coat the 96-well plate, 50 μL / well, and wrap at 4 °C. overnight; use blocking solution containing 5% skimmed milk powder for blocking; after washing, add the diluted serum to be tested, 50 μL / well, and react at 37°C for 2 hours; after washing, add enzyme-labeled secondary antibody, 50 μL / well, and incubate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com