2-(1-n-hexyloxy)ethylchlorin f salt, and pharmaceutical composition and application thereof
A technology of ethyl chlorin and n-hexyloxy, which is applied in the direction of drug combination, pharmaceutical formula, antineoplastic drugs, etc., can solve the problems of increasing the production cost of photosensitizers, and achieve short retention time, strong photodynamic activity, dark less toxic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
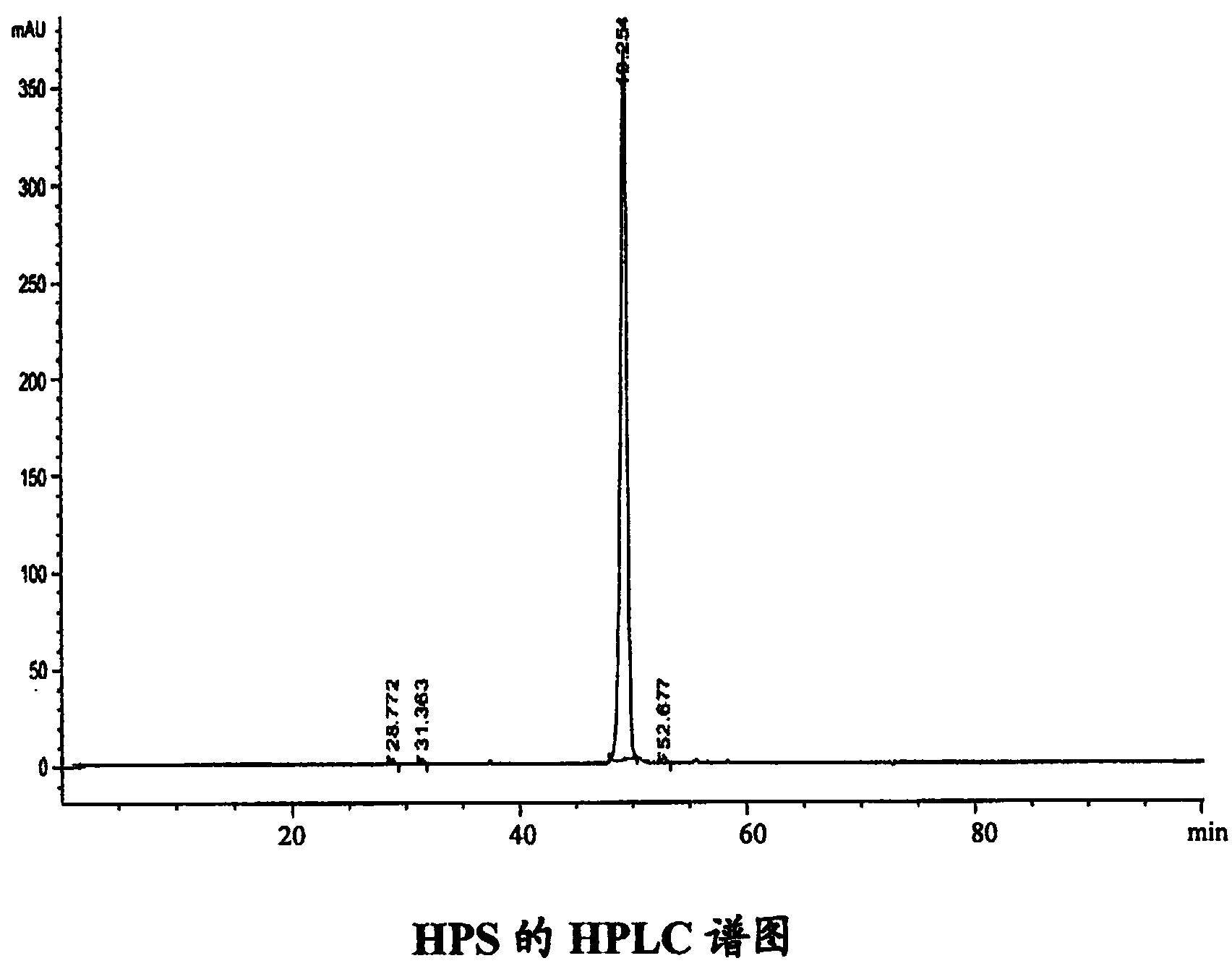
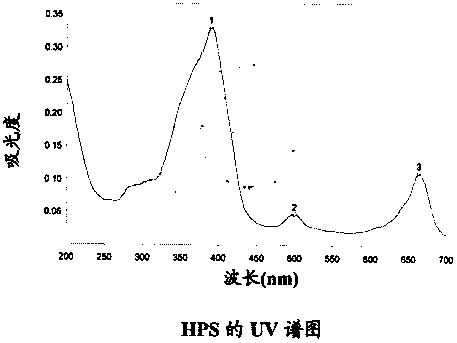
[0057] Embodiment 1: Preparation of 2-(1-hexyloxy)ethyl chlorin f sodium salt (HPS)
[0058] step 1.The preparation of the chlorin f of formula 2
[0059]
[0060] Dissolve 660mg of pheophorbide a methyl ester of formula 1 in pyridine (4ml), add 25% KOH-MeOH solution (50ml), and pass O at 0°C 2 reacted for 1 hour, and then introduced N 2 20 minutes, quickly boil and reflux for 40 minutes, cool slightly, add water (200ml), and use 10% H 2 SO 4 Neutralize to pH 5-6, filter with suction, and after the filter cake is dried, go through silica gel column chromatography (chloroform:methanol=20:1), separate and obtain the chlorin f of formula 2 (black powder, 70mg, yield 12%) .
[0061] Compound of formula 2: UV-vis[CH 3 OH,λ max (nm)(ε)]:662(121104),498(41964),398(436694); 1 H NMR (400MHz, DMSO-d 6 )δ:9.88(1H,s,β-H),9.85(1H,s,γ-H),9.74(1H,s,α-H),9.05(1H,s,δ-H),8.33(1H ,dd,J=18.0,11.6Hz,2a-H),6.45(1H,d,J=18.0Hz,2b-H B ),6.21(1H,d,J=12.0Hz,2b-H A ),4.63(1H,m,8-H),4.47...
Embodiment 2
[0071] Embodiment 2: Preparation of 2-(1-hexyloxy)ethyl chlorin potassium salt (HPK)
[0072] 2-(1-hexyloxy)ethyl chlorin potassium salt (HPK) was prepared in a similar manner to Example 1, except that in step 3, the isopropanol solution of potassium hydroxide was used to replace Sodium hydroxide solution in isopropanol.
[0073] HR-ESI-MS m / z718.00945 gives quasi-molecular ion peak [M+H] + , corresponding to the formula C 38 h 47 N 4 o 5 K 2 (calculated value 718.00833), with an unsaturation of 16.5.
[0074]
Embodiment 3
[0075] Example 3: Activity of Compounds of the Invention as Photosensitizers
[0076] The resuscitated mouse sarcoma S180 cells were inoculated into the peritoneal cavity of female Kunming mice. After 2-3 generations of passage, when the number of viable cells reached more than 90% (tested by trypan blue), they were collected from the peritoneal cavity of mice under sterile conditions. Extract the milky white tumor fluid, add normal saline, and dilute to a cell density of about 1×10 7 Tumor cell suspension, set aside.
[0077] Another 30 female Kunming mice were taken, and 0.2ml of tumor cell suspension was inoculated subcutaneously on the right shoulder blade of each mouse. 6 mice in each group were randomly divided into 5 groups, and 48 hours after inoculation, the mice were administered separately from the tail vein of each group.
[0078] Blank control group (normal saline);
[0079] Positive control group (HPPH0.3mg / kg);
[0080] High dose group (HPS0.32mg / kg);
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com