A kind of compound methoxyphenamine capsule and preparation method thereof
A technology of methoxyphenamine capsules and methoxyphenamine hydrochloride, which is applied in capsule delivery, pharmaceutical formulations, medical preparations of non-effective ingredients, etc., and can solve problems such as unrepresentative, over-limit, troubles in production and use, etc. , to achieve the effect of improving the stability of the preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Process Screening for Preparation of Compound Methoxyphenamine Capsules
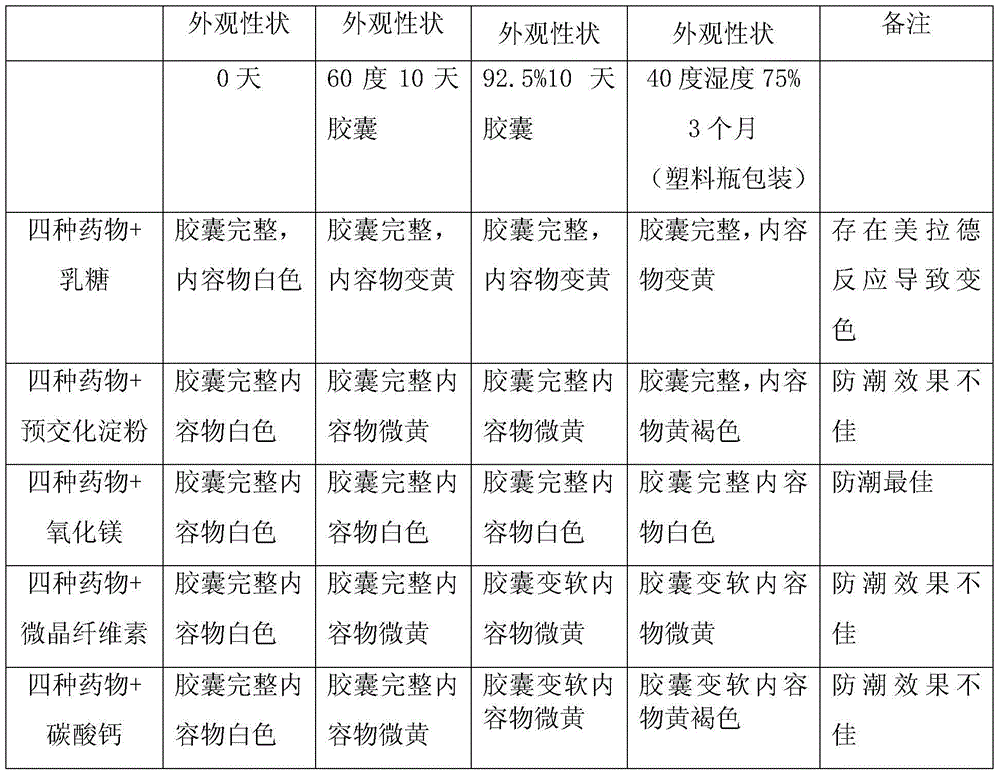
[0013] 1. Diluent screening
[0014] Table 1 Diluent Screening
[0015]
[0016] Magnesium oxide is selected as the diluent to solve the problem of strong hygroscopicity and easy discoloration and degradation of the drug.
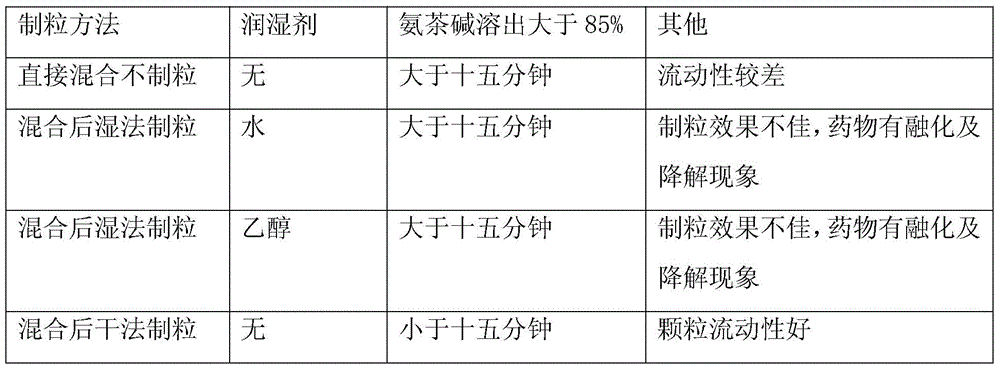
[0017] 2. Granulation process screening
[0018] Table 2 Screening of granulation process
[0019]
[0020] Based on the test results, dry granulation was selected as the granulation method to improve fluidity and solve the problems of strong hygroscopicity and easy discoloration and degradation of the drug.
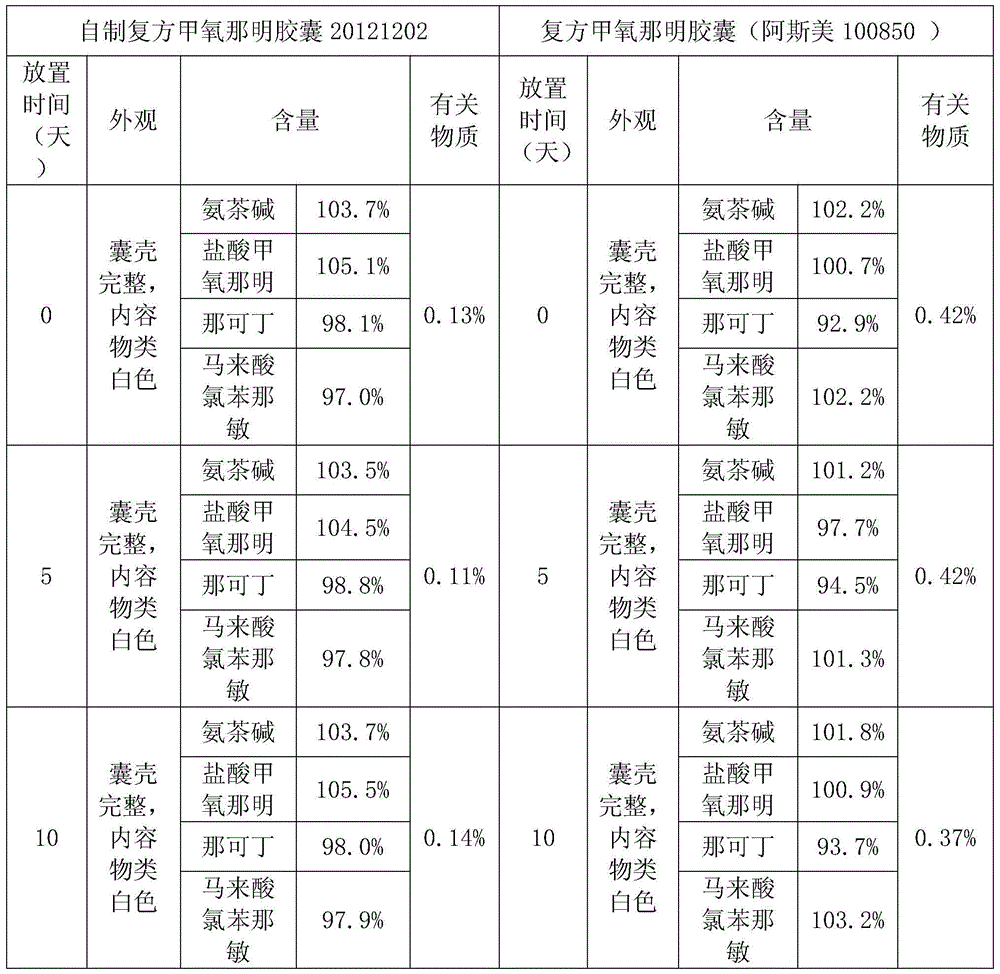
Embodiment 2
[0022] Crush all the drugs including methoxypheniramine hydrochloride, narcodine, aminophylline, and chlorpheniramine maleate respectively, and take 7 mg of narcodine, 2 mg of chlorpheniramine maleate, and sodium bisulfite 0.465mg, 0.465mg of sodium lauryl sulfate and 0.93mg of croscarmellose sodium are mixed evenly, then add 12.5mg of methoxyphenamine hydrochloride and mix evenly, then add 25mg of aminophylline and mix evenly, and finally add magnesium oxide Mix 32.55 mg with 0.465 mg magnesium stearate, dry granulate, pass through a 40-mesh sieve, add 9.3 mg magnesium aluminum silicate, mix evenly, and fill No. 5 capsules.
Embodiment 3
[0024] Grind all the drugs including methoxyphenamine hydrochloride, narcodine, aminophylline, chlorpheniramine maleate, and take the conventional prescription amount of methoxyphenamine hydrochloride 12.5mg, aminophylline 25mg, narcodine 7mg, horse Chlorpheniramine toate 2mg mix well. Add 2.325mg of sodium metabisulfite, 4.65mg of sodium dodecylbenzenesulfonate and 9.3mg of croscarmellose sodium starch and mix well, then add and mix well, and finally add 162.75mg of magnesium oxide and 0.465mg of micropowder silica gel and mix well, dry method granules, pass through a 40-mesh sieve, add 4.65 mg of magnesium aluminum silicate, mix evenly and fill No. 2 capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com