Paracetamol, aminophenazone, caffeine and chlorphenamine maleate tablet and preparation method thereof
A technology of phenamine and coffee sensitivity and prescription, which is applied in the field of medicine, can solve the problems of dust explosion safety hazards, increase the dust concentration in production workshops, and is not suitable for industrial scale-up production, and achieve the effect of simple operation and less dust
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
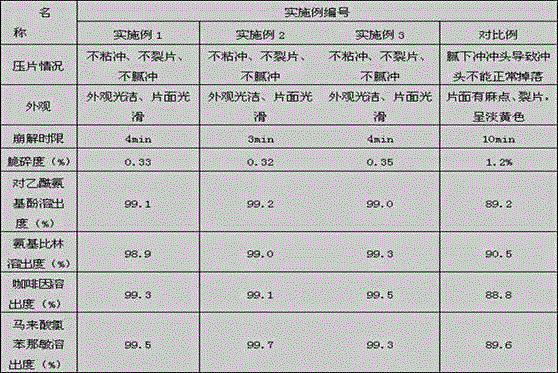
Embodiment 1
[0020] The prescription composition of embodiment 1 phenacamine tablets and its preparation method
[0021] Acetaminophen 150g
[0022] Aminopyrine 100g
[0023] Caffeine 30g
[0024] Chlorpheniramine Maleate 2g
[0025] Micronized silica gel 45g
[0026] 20g pregelatinized starch
[0027] Carboxypropyl cellulose 10g
[0028] Sodium Carboxymethyl Cellulose 40g
[0029] Sucrose 10g
[0030] Hypromellose 6.25g
[0032] Preparation method: grind aminopyrine and anhydrous caffeine respectively and pass through an 80-mesh sieve; add 125 g of purified water to hypromellose and sucrose, and prepare a mixed aqueous solution of 5% hypromellose and 8% sucrose; Acetaminophen 150g, caffeine 18g, chlorpheniramine maleate 1.2g, micropowder silica gel 18g, pregelatinized starch 20g, add 5% hypromellose, 8% sucrose mixed aqueous solution to make 16 mesh wet granules , dry the granules at 80°C; add 5% hypromellose, 8%...
Embodiment 2
[0033] Embodiment 2 The prescription composition of phenacamine tablet and its preparation method
[0034] Acetaminophen 150g
[0035] Aminopyrine 100g
[0036] Caffeine 30g
[0037] Chlorpheniramine Maleate 2g
[0038] Micronized silica gel 30g
[0039] 25g pregelatinized starch
[0040] Croscarmellose Sodium 30g
[0041] Sucrose 17g
[0043]Preparation method: Grind aminopyrine and anhydrous caffeine respectively and pass through 80-mesh sieve; add sucrose to purified water to prepare 8% sucrose mixed aqueous solution, then take 150g of paracetamol, 18g of caffeine, and chlorobenzene maleate Namin 1.2g, micropowder silica gel 12g, pregelatinized starch 15g, 8% sucrose mixed aqueous solution to make 24 mesh wet granules, temperature 80 ℃, dry granules; aminopyrine 100g, caffeine 12g, chlorobenzene maleate Namin 0.8g, micropowder silica gel 12g, pregelatinized starch 10g, and 8% sucrose mixed aqueous solution ar...
Embodiment 3
[0044] Embodiment 3 The prescription composition of phenacamine tablet and its preparation method
[0045] Acetaminophen 150g
[0046] Aminopyrine 100g
[0047] Caffeine 30g
[0048] Chlorpheniramine Maleate 2g
[0049] Micronized silica gel 50g
[0050] Carboxypropyl cellulose 40g
[0051] Crospovidone 40g
[0052] Hypromellose 15g
[0054] Preparation method: Grind aminopyrine, acetaminophen, and anhydrous caffeine respectively and pass through an 80-mesh sieve; add hypromellose and purified water to prepare a 5% hypromellose aqueous solution; take paracetamol Phenol 150g, caffeine 18g, chlorpheniramine maleate 1.2g, micropowder silica gel 20g, carboxypropyl cellulose 24g, add 5% hypromellose aqueous solution to make 48 mesh wet granules, temperature 80℃, dry granules ; Aminopyrine 100g, caffeine 12g, chlorpheniramine maleate 0.8g, micropowder silica gel 20g, carboxypropyl cellulose 16g, add 5% hypromellose ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com