Redox-sensitive hyaluronic acid-docetaxel conjugate and preparation method thereof
A technology of docetaxel and hyaluronic acid, which is applied in the field of hyaluronic acid-docetaxel conjugates and its preparation, can solve problems such as occurrence, and achieve the effects of improving solubility, simple preparation method, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
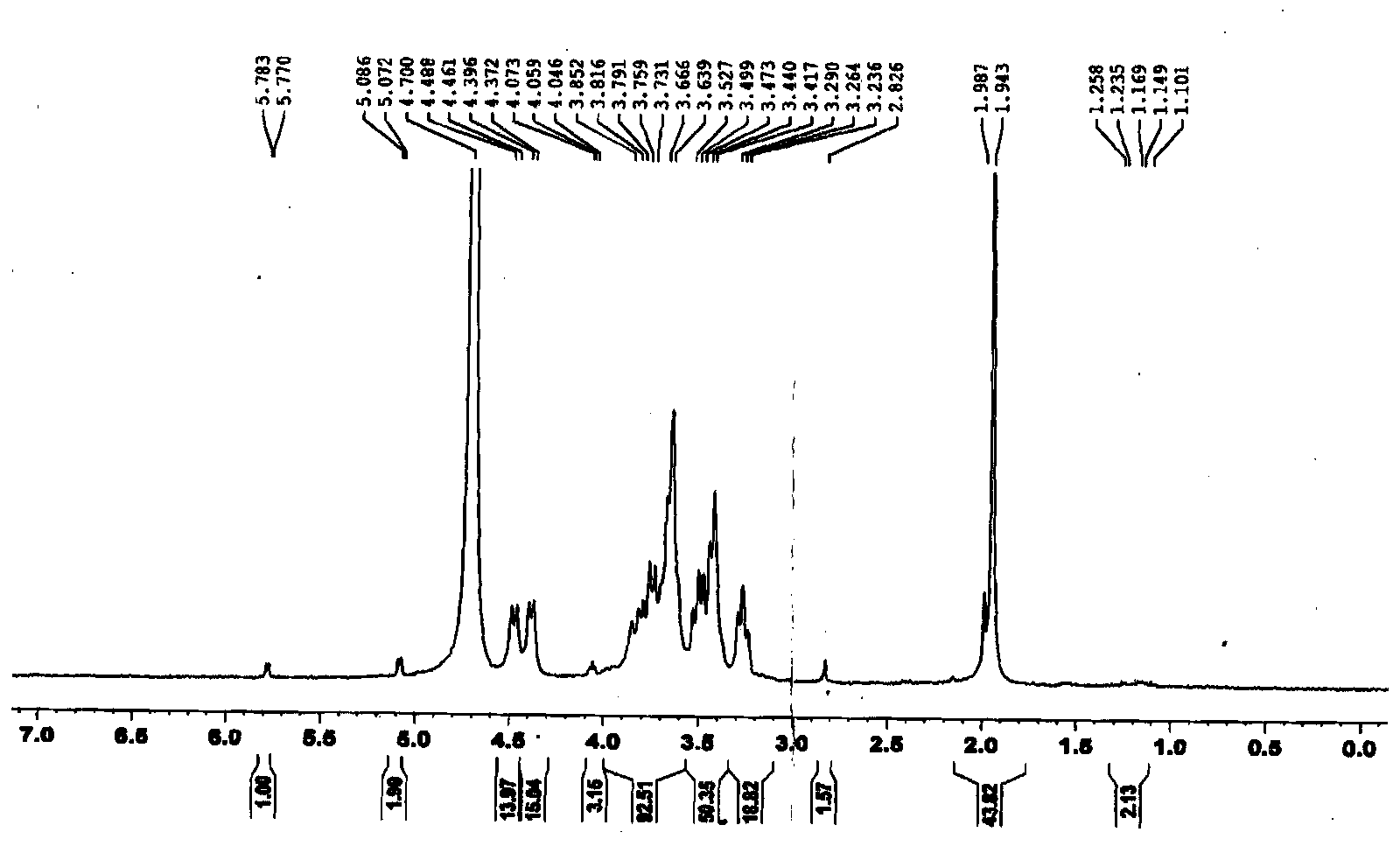
[0041] Example 1: Preparation of redox-sensitive hyaluronic acid-docetaxel conjugates
[0042] Proceed as follows:
[0043] Weigh 300 mg of docetaxel, mix it with 2 mg of succinic anhydride, dissolve it in 4 mL of dichloromethane, add 60 μL of pyridine, stir at room temperature for 72 h, and vacuum rotary evaporate (remove dichloromethane). Purified to obtain 200 mg of docetaxel succinic anhydride derivative. Dissolve 120 mg of docetaxel succinic anhydride derivative in 4 mL of N,N-dimethylformamide, add 45 mg of N-hydroxysuccinimide and 824 mg of 1-ethyl-(3-dimethylaminopropyl) carbon The imide hydrochloride was activated at room temperature to obtain 130 mg of succinic anhydride activated ester of docetaxel.
[0044]Dissolve 3 g of hyaluronic acid in 1 mL of PBS buffer (pH 7.4), add 18 mg of 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride and 300 mg of thiolated N-hydroxybutyl The diimide is activated by stirring. After activation, 10 g of cystamine hydrochlor...
Embodiment 2
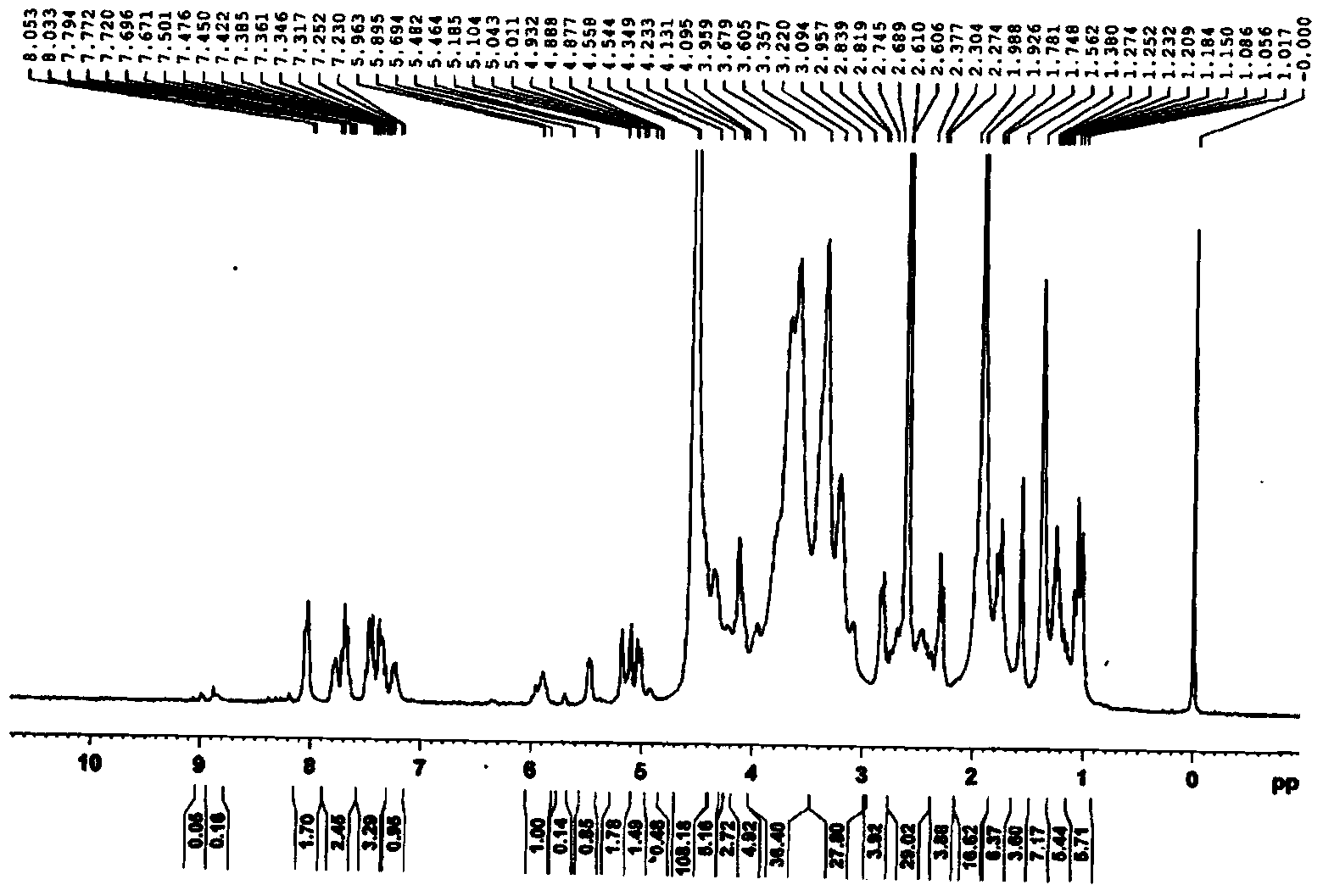
[0046] Example 2: Preparation of redox-sensitive hyaluronic acid-docetaxel conjugates
[0047] Proceed as follows:
[0048] Weigh 270 mg of docetaxel, mix it with 14 mg of succinic anhydride, dissolve it in 3 mL of dichloromethane, add 124 μL of pyridine, stir at room temperature for three days, and vacuum rotary evaporate (remove dichloromethane). Purified to obtain 250 mg of docetaxel succinic anhydride derivative. Dissolve 200 mg of docetaxel succinic anhydride derivative in 5 mL of N,N-dimethylformamide, add 68 mg of N-hydroxysuccinimide and 24 mg of 1-ethyl-(3-dimethylaminopropyl) carbon The imide hydrochloride was activated at room temperature to give 230 mg of the succinic anhydride activated ester of docetaxel.
[0049] Dissolve 3.2 g of hyaluronic acid in 4 mL of MES buffer (pH 4.75), add 48 mg of 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride and 27 mg of thiolated N-hydroxy Succinimide is activated by stirring. After activation, add 10 g of cystamine...
Embodiment 3
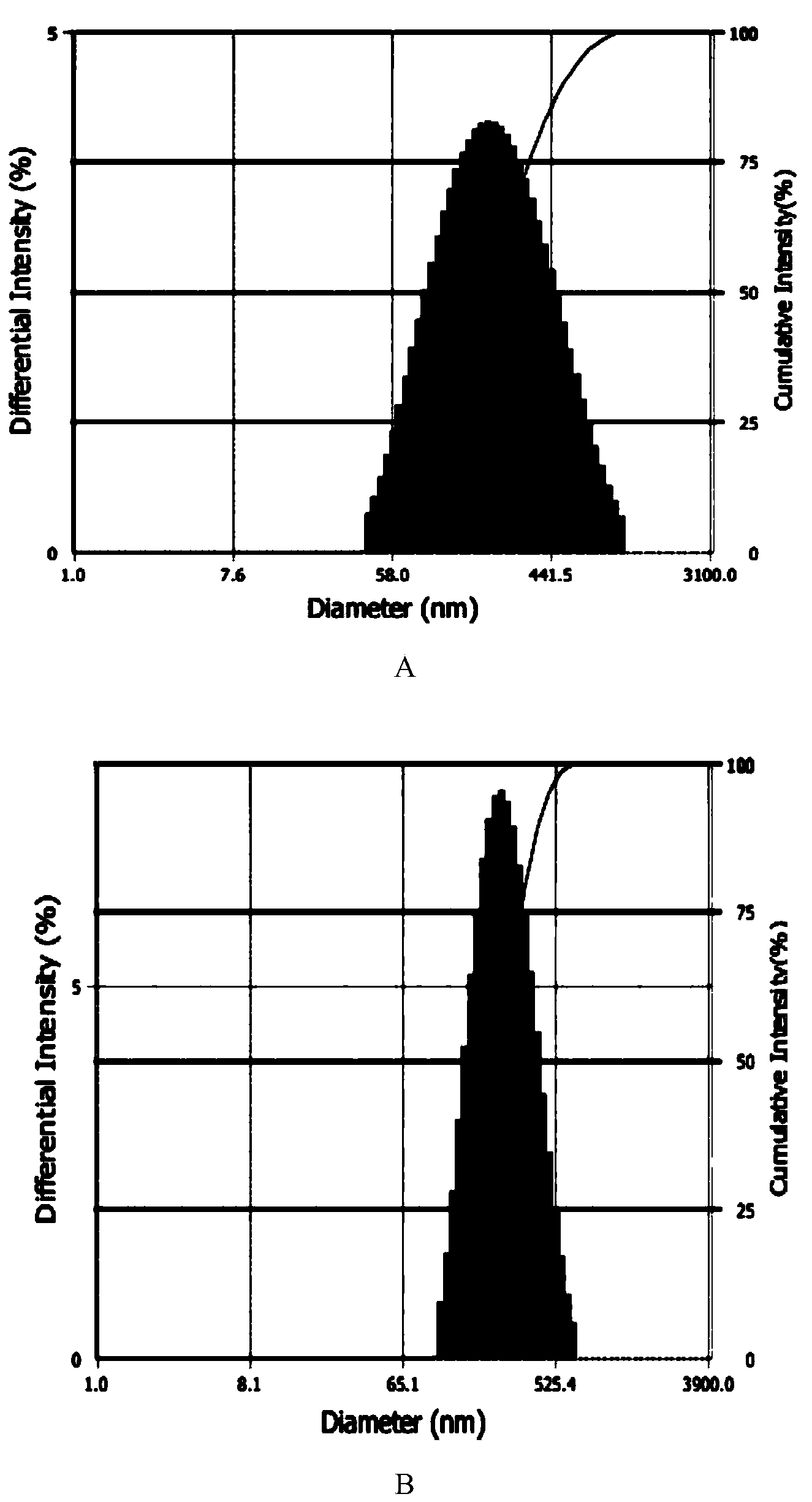
[0050] Example 3: Preparation of redox-sensitive hyaluronic acid-docetaxel conjugates
[0051] Proceed as follows:
[0052] Weigh 34 mg of docetaxel, mix with 12.5 mg of succinic anhydride, dissolve in 2 mL of dimethyl sulfoxide, add 0.565 mL of pyridine, stir at room temperature for 72 h, and vacuum rotary evaporate (remove dimethyl sulfoxide). Purified to obtain 30mg of docetaxel succinic anhydride derivative. Dissolve 20 mg of docetaxel succinic anhydride derivative in 5 mL of N,N-dimethylformamide, add 23 mg of N-hydroxysuccinimide and 76 mg of 1-ethyl-(3-dimethylaminopropyl) carbon The imide hydrochloride was activated at room temperature to give 25 mg of the succinic anhydride activated ester of docetaxel.
[0053] Dissolve 500mg of hyaluronic acid in 10mL of MES buffer (pH 4.75), add 48mg of 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride and 27mg of thiolated N-hydroxybutyl The diimide is activated by stirring. After activation, adjust the pH to 7.4 with ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Electric potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com