Method of extracting and separating costunolide and dehydrocostuslactone
A technology of dehydrocosmolide and cosmolide, which is applied in the production of bulk chemicals and organic chemistry, and can solve the problems of complex solvent types, cumbersome operation steps, and long separation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1 Extraction and separation method of the present invention
[0033] A. Preparation of solvent system: Prepare an appropriate amount of solution according to the ratio of petroleum ether-ethanol-water (5:7:3) at room temperature, shake it in a separatory funnel, and let it stand for stratification. The upper phase is used as the stationary phase, and the lower phase is used as the mobile phase. Mutually.
[0034]B. Sample preparation: take 100g of woody herbs (passed through a No. 3 sieve), add 500ml of petroleum ether, soak overnight, and ultrasonically extract for 30 minutes. Extracted twice in the same way. The extracts were combined, filtered, and the filtrate was spun to dryness to obtain 3.8 g of crude extract of Akira sinensis. Take 100mg of crude extract of Akira sinensis and add 20ml of mobile phase to fully dissolve the sample.
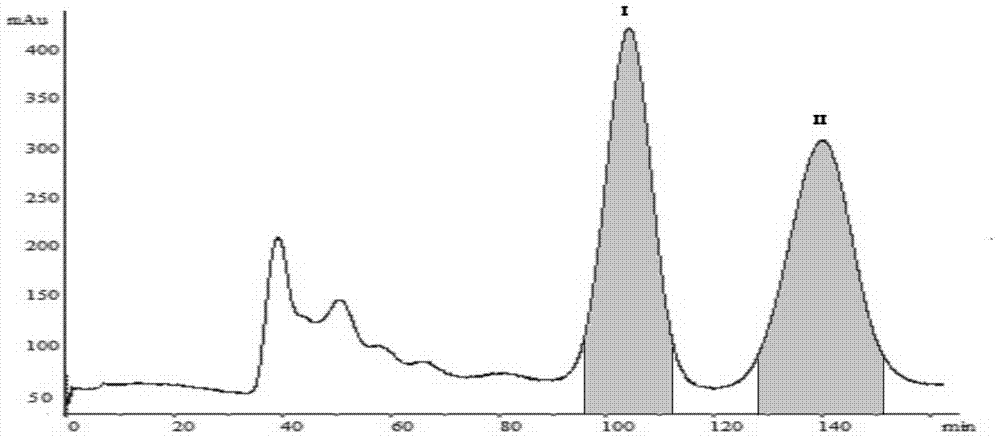
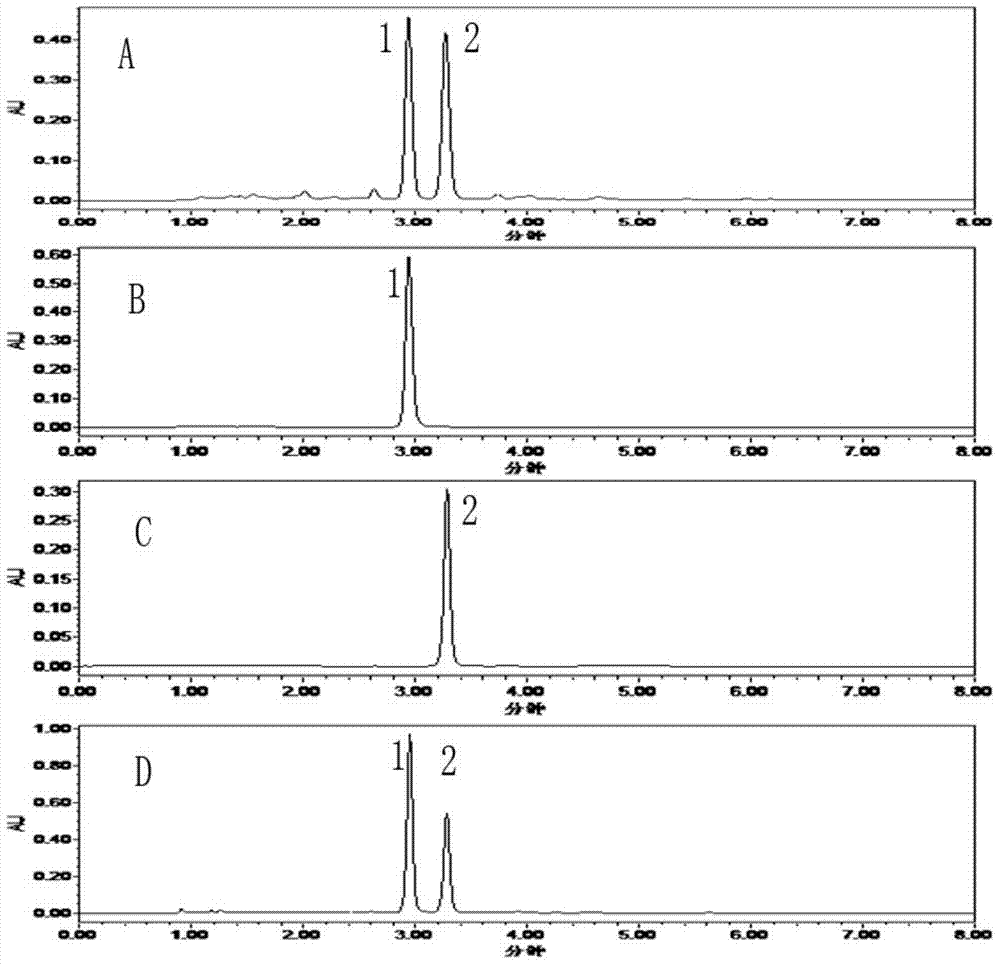
[0035] C. Separation of HSCCC by high-speed countercurrent chromatography: Fill the chromatographic column with the st...
Embodiment 2
[0039] Embodiment 2 Extraction separation method of the present invention
[0040] A. The preparation of the solvent system: prepare an appropriate amount of solution according to the ratio of petroleum ether-ethanol-water (5:5:2.5) at room temperature, shake it in a separatory funnel, and let it stand for stratification. The upper phase is used as the stationary phase, and the lower phase is used as the mobile phase. Mutually.
[0041] B. Sample preparation: Take 200mg of the crude extract of Akira sinensis and add 20ml of mobile phase to fully dissolve the sample.
[0042] C. Separation of HSCCC by high-speed countercurrent chromatography: Fill the chromatographic column with the stationary phase (upper phase) from the head end to the tail end, the main engine rotates forward at a speed of 900r / min, and then pump the mobile phase (lower phase) at a flow rate of 2ml / min , when the mobile phase flows out and the two-phase solvent reaches equilibrium in the column, the retenti...
Embodiment 3
[0044] Embodiment 3 Extraction separation method of the present invention
[0045] A. Preparation of solvent system: Prepare an appropriate amount of solution according to the ratio of petroleum ether-ethanol-water (5:5:5) at room temperature, shake it in a separatory funnel, and let it stand for stratification. The upper phase is used as the stationary phase, and the lower phase is used as the mobile phase. Mutually.
[0046] B. Sample preparation: take 100g of woody herbs (passed through a No. 3 sieve), add 500ml of petroleum ether, soak overnight, and ultrasonically extract for 30 minutes. Extracted twice in the same way. The extracts were combined, filtered, and the filtrate was spun to dryness to obtain 3.8 g of crude extract of Akira sinensis. Take 100mg of crude extract of Akira sinensis and add 20ml of mobile phase to fully dissolve the sample.
[0047] C. Separation of HSCCC by high-speed countercurrent chromatography: Fill the chromatographic column with the stati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com