Cefoperazone sodium compound entity, composition and application
A kind of technology of cefoperazone sodium and compound, which is applied to the new compound entity and composition of cefoperazone sodium and the field of preparation thereof, and achieves the effects of improving operability, good sliding property, convenient storage and transportation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
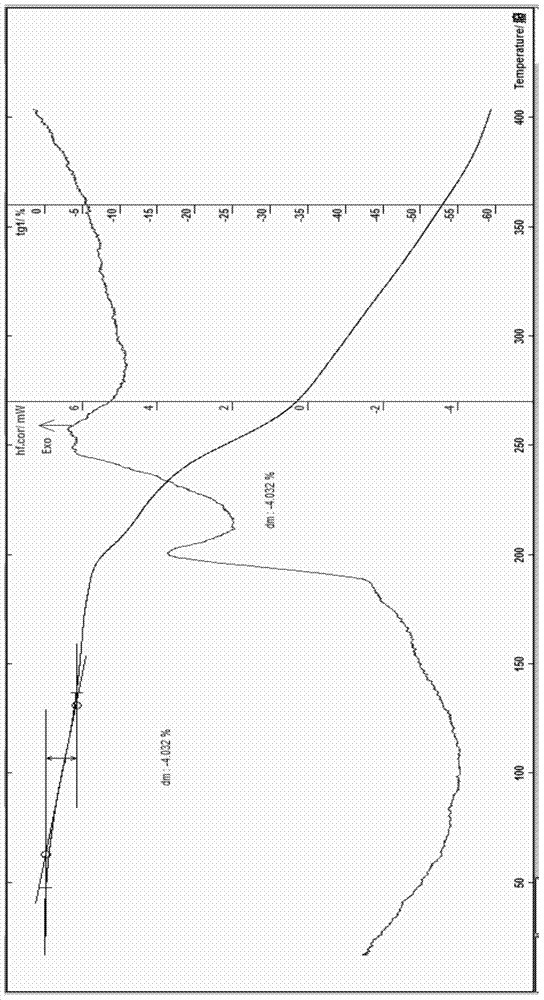
[0063] The preparation of embodiment 1 cefoperazone sodium 1.5 hydrate
[0064] Add 10g of cefoperazone acid, 85m of ethanol, and 3ml of water into a 500ml flask, stir to dissolve, add 0.1g of activated carbon, stir for 30 minutes, filter with suction, add a mixed solution of sodium carbonate and ethanol dropwise to the filtrate at 5°C, and stir , adjust the pH to about 7.0 with glacial acetic acid, slowly add 50ml of acetone and 250ml of ethanol dropwise, and place it below -4°C to allow the solid to fully separate out, filter with suction, wash with a small amount of cold ethanol and chloroform for 3 times, filter with suction, and dissolve the obtained solid with an appropriate amount of water , Add 240ml of ethanol and 30ml of acetone as the crystallization solvent for recrystallization, place it below 5°C for about 2 hours, then place it at 0~-4°C for about 3 hours, and place it at about -10°C overnight, so that the crystals can be fully analyzed. Filter, wash with a smal...
Embodiment 2
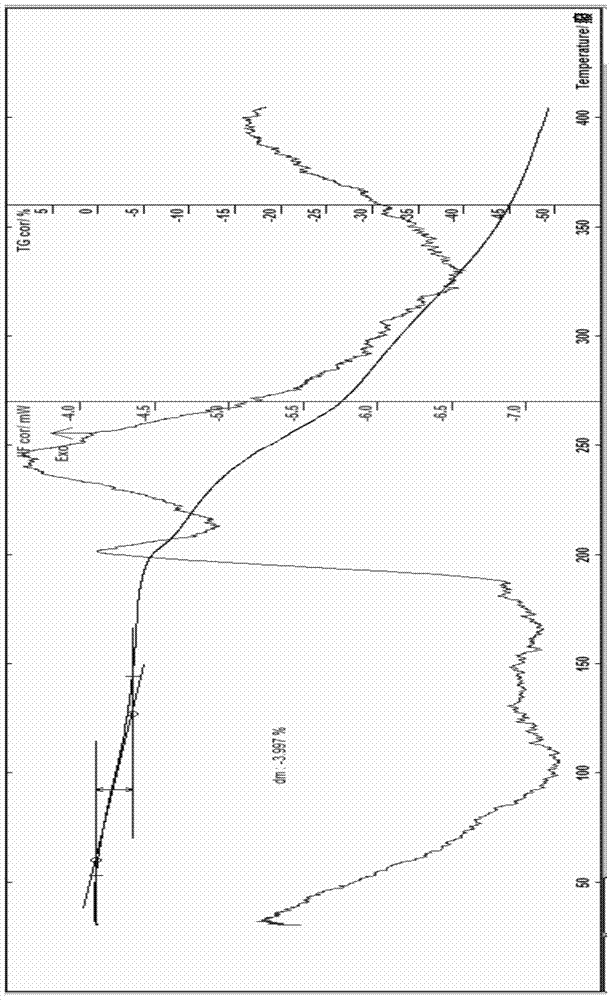
[0065] The preparation of embodiment 2 cefoperazone sodium 1.5 hydrate
[0066] Add 20g of cefoperazone acid and 50ml of water into a 250ml flask, stir to form a suspension, add dropwise 20ml of an aqueous solution of 2.9g of anhydrous sodium carbonate at 15°C, stir to dissolve, add 0.2g of activated carbon, stir for 30 minutes, and pump Filtrate, wash with water, and suction filter, adjust the pH of the filtrate to about 7.2 with glacial acetic acid, then slowly add 100ml of acetone, 250ml of ethanol, and 5ml of isopropyl ether dropwise, and place it below 0°C to allow the solids to fully separate out, then filter with suction, and add a small amount of ethanol, acetone and Wash 3 times with chloroform, filter with suction, dissolve the obtained solid with an appropriate amount of water, use 80ml of acetone, 230ml of ethanol, 10ml of isopropanol, and 5ml of isopropyl ether as the crystallization solvent for recrystallization, place it below 5°C for about 3 hours, and then plac...
Embodiment 3
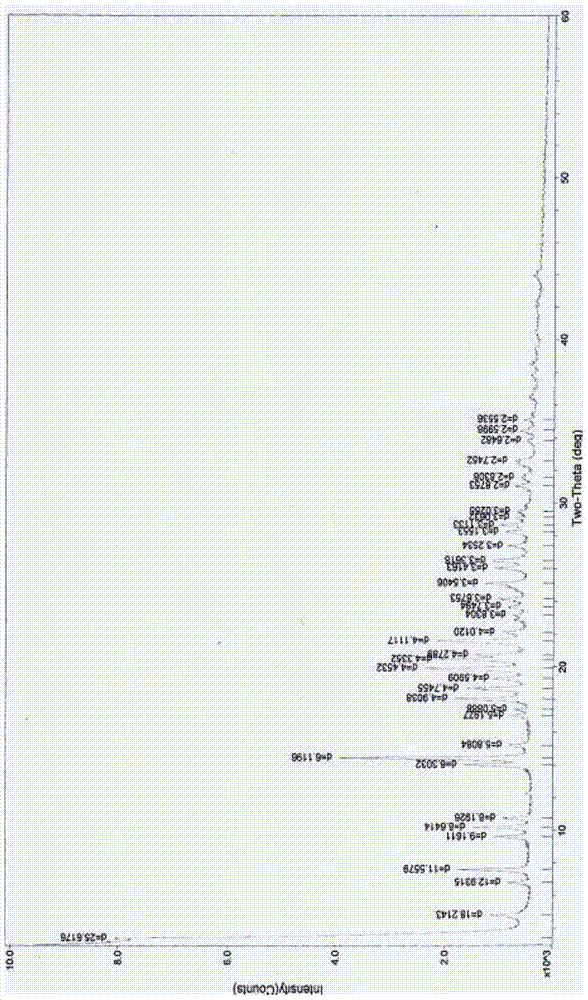
[0067] The preparation of embodiment 3 cefoperazone sodium 2 hydrates
[0068] Add 10g of cefoperazone acid and 30ml of water into a 250ml flask, stir to form a suspension, add a saturated aqueous solution of sodium bicarbonate dropwise at 10°C until the pH is about 7.0, stir to dissolve, add 0.2g of activated carbon, and stir for 30 minutes. Suction filtration, washing with water, suction filtration, slowly dropwise add 10ml of chloroform, 300ml of acetone, 20ml of isopropanol to the filtrate and place it below 10°C to allow the solid to be fully separated out, suction filtration, washing with a small amount of ethanol for 3 times, suction filtration, and the obtained solid with an appropriate amount of Dissolve in water, 300ml of acetone, 10ml of chloroform, 20ml of isopropanol as crystallization solvent for recrystallization, place at about 8°C for about 2 hours, then place at -4°C for about 3 hours, and place at -15°C for about 10 hours , the crystals were fully separated,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com