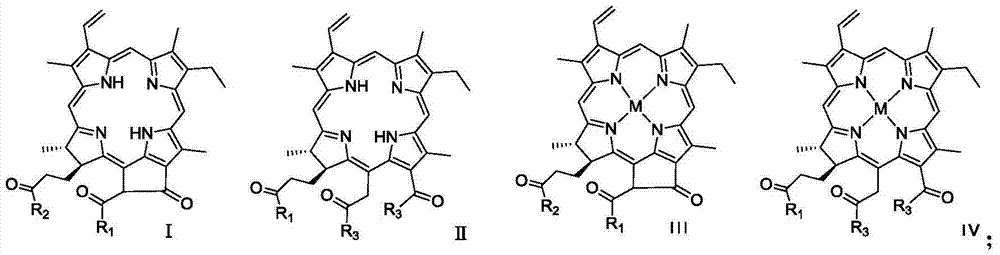
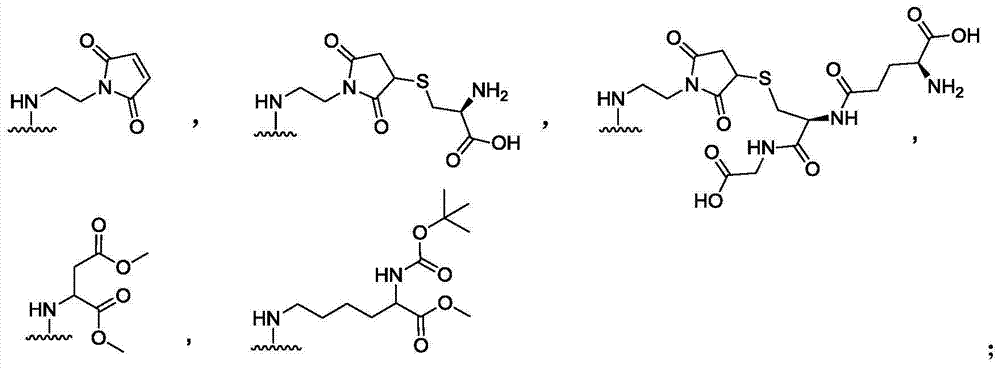
Dihydroporphine photosensitizer and sonosensitizer and preparation method and application thereof
A technology of chlorin and sound sensitizer, which is applied in the field of chemical medicine and can solve the problems of slow clearance rate, poor solubility, and large dose required
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Synthesis of compound a
[0027] Dissolve 100mg of commercially available compound 1 in 10mL of dichloromethane and 2mL of DMF, add 25.1mg of HOBt, 59.6mg of TBTU, and 500μL of DIEA in sequence, stir at 0-50°C, protect with nitrogen balloon, TLC (monitoring, stop after 30min) Then add 49.2mg N-(2-aminoethyl)maleimide, 500μL DIEA, 1mL DMF, stir at 0-50℃, protect with nitrogen balloon, monitor by TLC, and stop the reaction after 6h. Dilute with methyl chloride, place in a 250mL separatory funnel, wash with deionized water (50mL×3), dry the dichloromethane layer with anhydrous sodium sulfate, and concentrate to obtain a crude product, which is subjected to silica gel column chromatography (developing solvent: Petroleum ether: ethyl acetate = 1:4) purification to obtain 57.7 mg of dark green solid, namely compound a. ESI-MS m / z: 715.3 [M+H] + . 1 H NMR (400MHz, CDCl 3 )δ9.32(1H,s),9.29(1H,s),8.54(1H,s),7.97(1H,dd,J=17.2,10.5Hz,),6.58(2H,s),6.27(1H, dd,J=17.2,1.2Hz),6.16...
Embodiment 2
[0033] Synthesis of compound d
[0034] Dissolve 40.1 mg of commercially available compound 2 in 10 mL of DMF, add 23.3 mg of N-(2-aminoethyl)maleimide, 1 mL of triethylamine, stir at 0-50°C, protect with nitrogen balloon, monitor by TLC, The reaction was stopped after 2h. Add 30.2 mg of anhydrous potassium carbonate and 230 μL of methyl iodide to the reaction solution, continue to stir at a temperature of 0 to 50 ° C, protect with a nitrogen balloon, and detect the reaction by TLC (developer: petroleum ether: ethyl acetate = 1:2), and the reaction proceeds. Stop after 2h. The reaction solution was diluted with 40mL of dichloromethane, transferred to a 250mL separatory funnel, washed with deionized water (50mL×3), washed with saturated brine (50mL×2), dried over anhydrous sodium sulfate, filtered with suction, and concentrated the organic layer to obtain The crude product was purified by silica gel column chromatography (petroleum ether: ethyl acetate = 1:2) to obtain 36.4 m...
Embodiment 3
[0040] Synthesis of compound g
[0041] ① Dissolve 53.2 mg of commercially available compound 2 in 10 mL of methanol, add 200 μL of concentrated sulfuric acid dropwise, stir at 0-50 °C, protect with nitrogen balloon, monitor by TLC, stop the reaction after 4 hours, add 20 mL of deionized water to the reaction solution, and Transfer to a 125mL separatory funnel, extract with dichloromethane (30ml×3), combine the dichloromethane layers, add anhydrous sodium sulfate to dry, filter with suction, and concentrate to obtain the crude product. The crude product was purified by silica gel column chromatography (petroleum ether: ethyl acetate = 1:4) to obtain 44.2 mg of a dark green solid, compound 3, with a yield of 77%. ESI-MS m / z:625.4[M+H] + . 1 H NMR (400MHz, CDCl 3 )δ9.64(1H,s),9.50(1H,s),8.72(1H,s),8.01(1H,dd,J=17.8,11.5Hz),6.32(1H,dd,J=17.8,1.2Hz ),6.12(1H,dd,J=11.5,1.2Hz),5.51(1H,d,J=18.7Hz),5.26(1H,d,J=18.7Hz),4.46(1H,q,J=7.56Hz ),4.12(1H,d,J=7.1Hz),3.82(3H,s),3.74(2H,m),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com