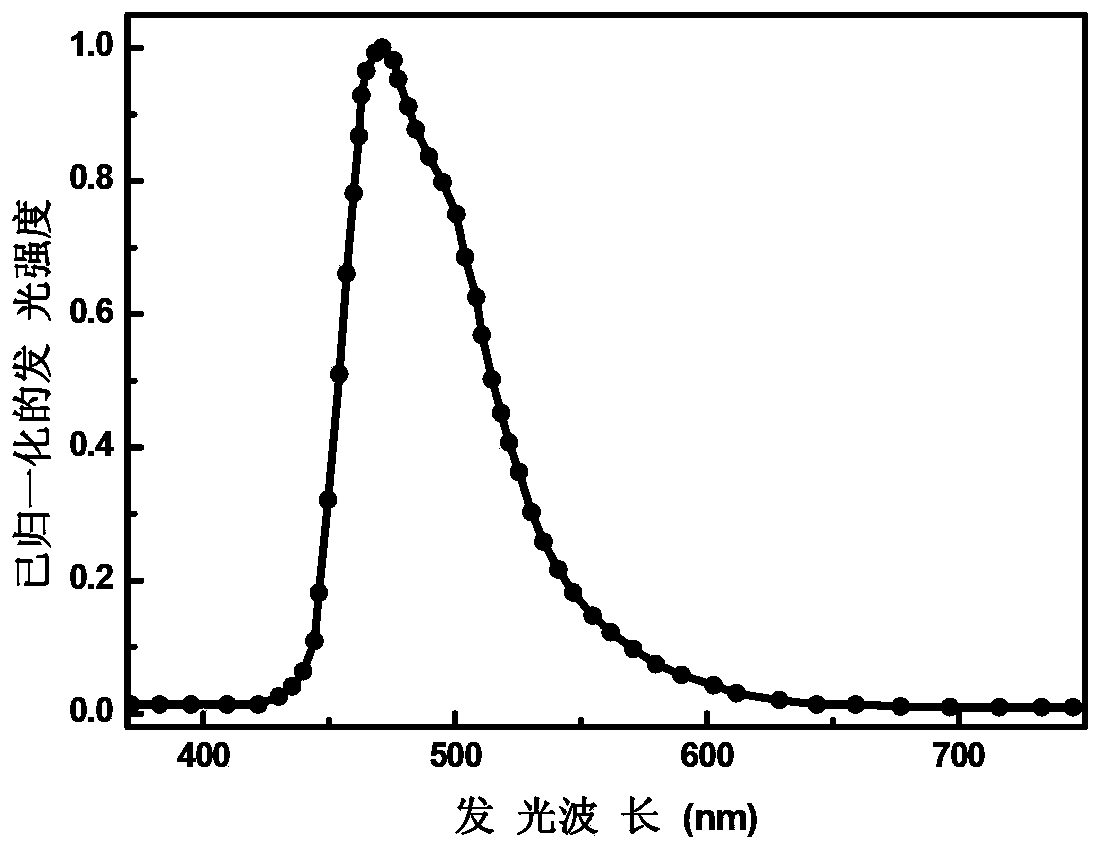
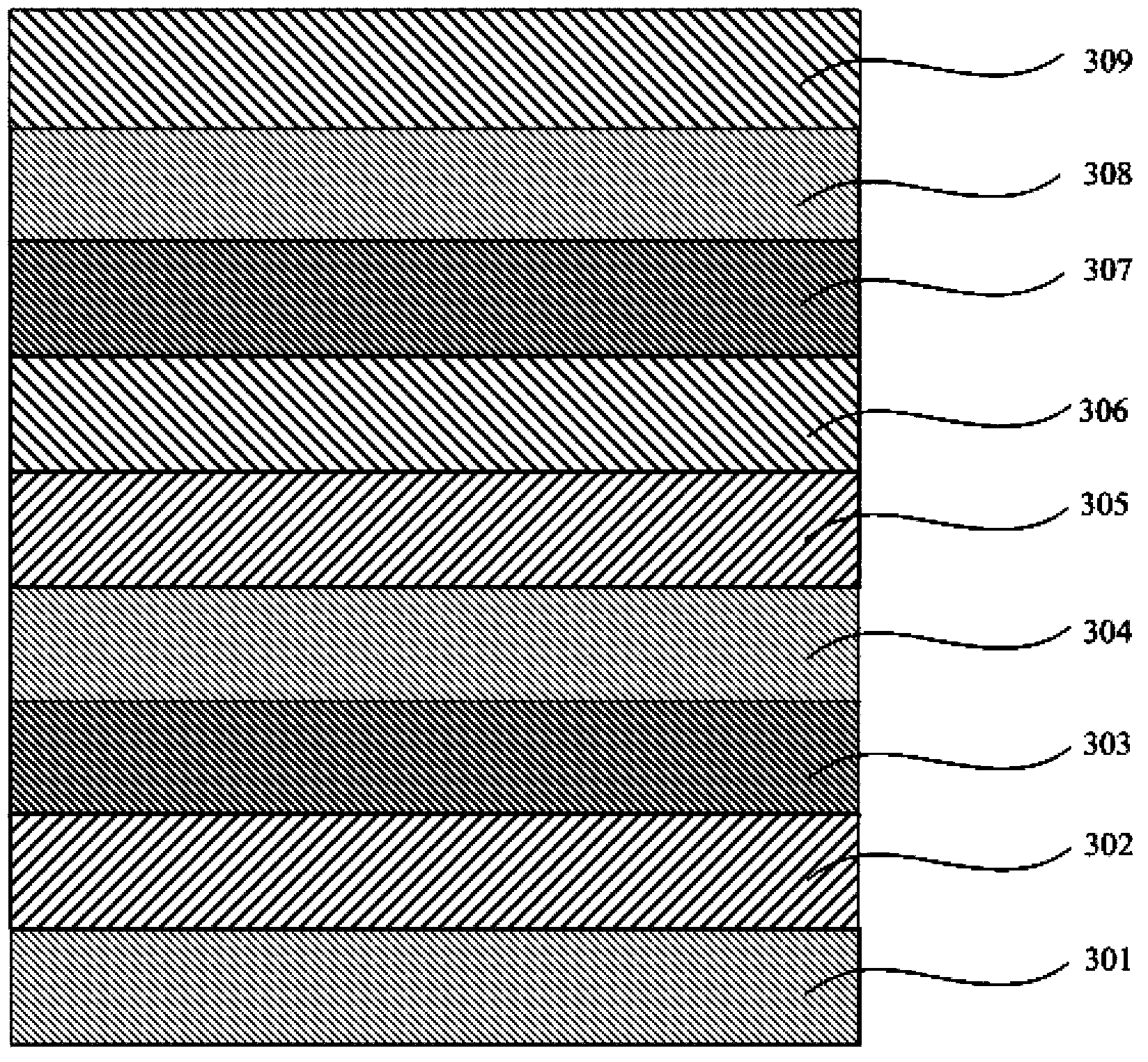
Organic electrophosphorescent material and preparation method thereof and organic light-emitting device
A phosphorescent material, electrophosphorescence technology, applied in luminescent materials, electro-solid devices, organic chemistry, etc., to achieve the effect of facilitating evaporation, reducing self-quenching phenomenon, and reducing direct effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The present invention also relates to a method for preparing the above-mentioned organic electrophosphorescent material, which method comprises the following steps in sequence:
[0038] 1. Synthesize compound C through Suzuki coupling reaction between compound E and compound F; wherein, compound F is 2,3,4-trifluorophenylboronic acid, and the structural formulas of compound E and compound C are as follows:
[0039] Compound E is Compound C is
[0040] 2. Reaction of compound C prepared in step 1 with compound D to generate a chlorine-bridged dimer, namely compound A. Wherein, compound D is trihydrate iridium trichloride IrCl 3 ·3H 2 O. The structural formula of compound A is as follows:
[0041]
[0042] 3. Use the compound A prepared in step 2 as the main structure of the ring metal ligand, and use 2,4-bis(trifluoromethyl)-5-(pyridin-2'-yl)pyrrole (compound B) as the auxiliary Ligand source, reacting compound A and compound B to obtain an iridium metal comp...
Embodiment 1
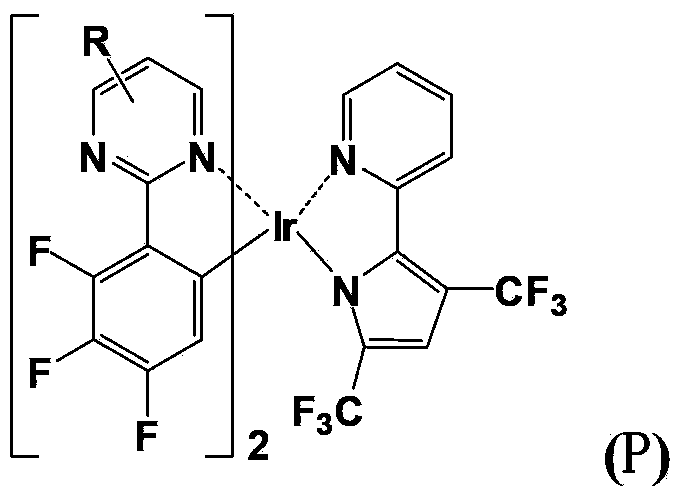
[0050] The organic electrophosphorescent material disclosed in this example is the complex bis(2-(4',5',6'-trifluorophenyl)-5-phenylpyrimidine-N,C 2 ')(2,4-bis(trifluoromethyl)-5-(pyridine-2'-yl)pyrrole) iridium, whose structural formula is as follows:
[0051]
[0052] It is prepared by the following steps:
[0053] (1) Synthesis of 2-(2',3',4'-trifluorophenyl)-5-phenylpyrimidine
[0054] The synthetic reaction formula is as follows:
[0055]
[0056] The specific steps are: under nitrogen atmosphere, mix 2.35g (10mmol) 2-bromo-5-phenylpyrimidine, 2.11g (12mmol) 2,3,4-trifluorophenylboronic acid and 0.58g (0.5mmol) tetrakis (tri Phenylphosphorus)palladium was dissolved in 50ml of toluene, and then 25ml of an aqueous solution containing 2.76g (20mmol) of potassium carbonate was added dropwise to the reaction system, heated to 100°C and stirred for 10 hours. After the reaction solution was cooled to room temperature, Dichloromethane extraction, liquid separation, washi...
Embodiment 2
[0072] The organic electrophosphorescent material disclosed in this example is the complex bis(2-(4',5',6'-trifluorophenyl)-4-(fluorene-9'-yl)pyrimidine-N,C 2 ')(2,4-bis(trifluoromethyl)-5-(pyridine-2'-yl)pyrrole) iridium, whose structural formula is as follows:
[0073]
[0074] It is prepared by the following steps:
[0075] (1) Complex bis(2-(4',5',6'-trifluorophenyl)-4-(fluoren-9'-yl)pyrimidine-N,C 2' ) (2-pyridinecarbonyl) iridium synthesis
[0076] The synthetic reaction formula is as follows:
[0077]
[0078] The specific steps are: under nitrogen atmosphere, 3.23g (10mmol) 2-bromo-4-(fluorene-9'-yl) pyrimidine, 1.76g (10mmol) 2,3,4-trifluorophenylboronic acid and 0.21g ( 0.3mmol) dichlorobis(triphenylphosphine)palladium is dissolved in 60ml of N,N-dimethylformamide (DMF), and then 30ml of aqueous solution containing 1.59g (15mmol) sodium carbonate is added dropwise to the reaction system , heated to 90° C. and stirred for 12 hours. After the reaction solutio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com