Tablet containing grifola frondosa and preparation method thereof
A technology of Grifola frondosa and Grifola frondosa polysaccharides, applied in the field of medicine, can solve the problems of not meeting the quality requirements of drugs, unqualified dissolution rate, fast moisture absorption of Grifola frondosa, etc., to ensure long-term stable storage, ensure consistency, improve The effect of formability and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
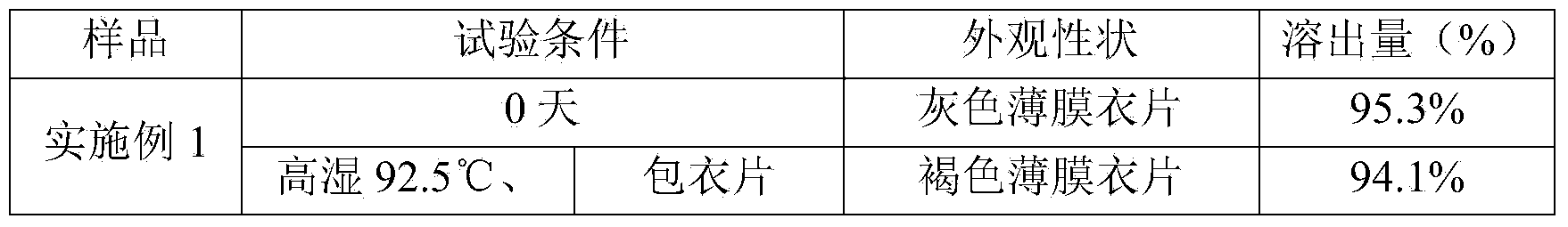
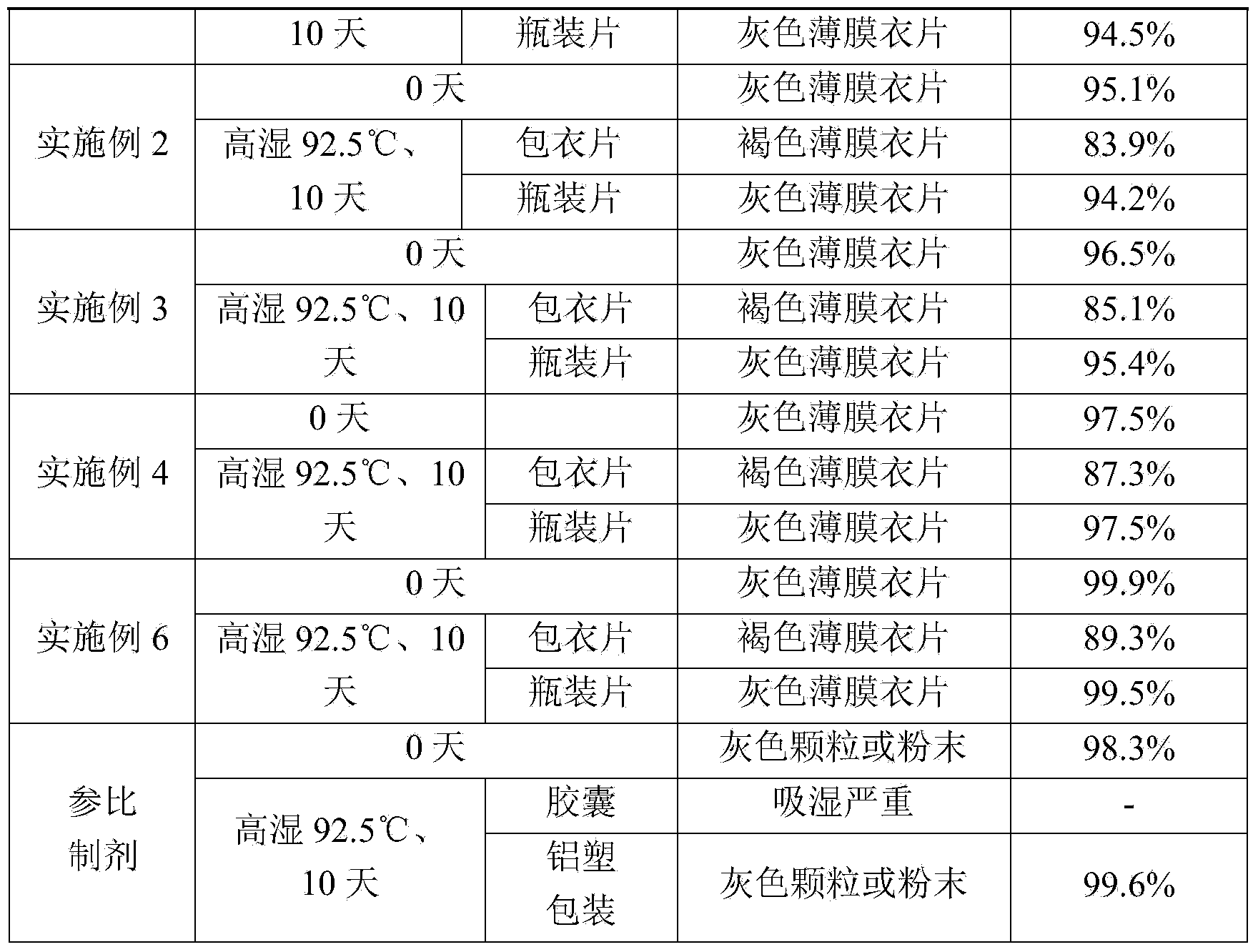
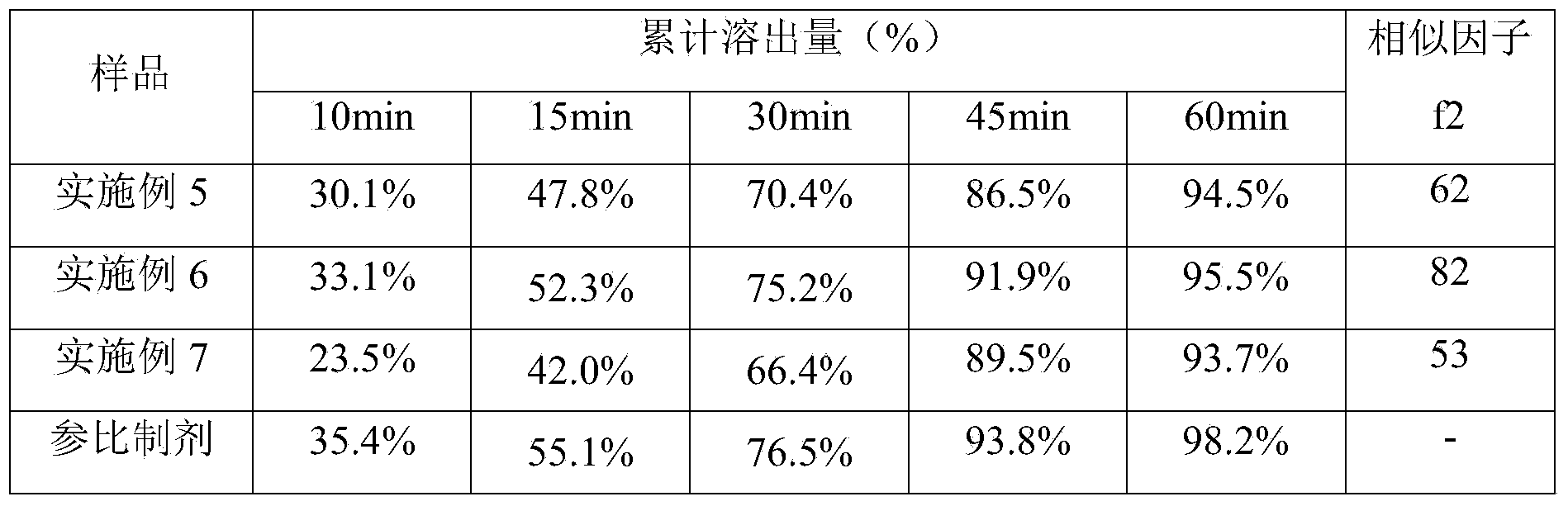
[0033] Accurately weigh: Grifola frondosa polysaccharide 500g, crospovidone 40g, magnesium stearate 5g, gastric soluble coating premix 25g. Grifola frondosa polysaccharide and crospovidone are pulverized, passed through a 80-mesh sieve, mixed and dry-granulated to obtain granules; added magnesium stearate that has been weighed, and mixed; then, under the condition that the ambient humidity is less than 45%. The next steps are: tableting, coating, and subpackaging to obtain 1000 tablets of Grifola frondosa.
Embodiment 2
[0035] Accurately weigh: Grifola frondosa 500g, crospovidone 60g, magnesium stearate 5g, silicon dioxide 5g, gastric soluble coating premix 25g. Grifola frondosa polysaccharide, crospovidone and silicon dioxide were pulverized, passed through an 80-mesh sieve, mixed and dry-granulated to obtain granules; Under the condition of less than 45%, sequentially: tableting, coating, sub-packaging, 1000 tablets of Grifola frondosa tablets are obtained.
Embodiment 3
[0037] Accurately weigh: Grifola frondosa 500g, copovidone S63040g, crospovidone 60g, magnesium stearate 5g, silicon dioxide 5g, sodium stearyl fumarate 5g, gastric soluble coating premix 35g. Grifola frondosa polysaccharide, crospovidone, copovidone S630, silicon dioxide, and sodium stearyl fumarate were pulverized, passed through an 80-mesh sieve, mixed and dry-granulated to obtain granules; Magnesium fatty acid, mixed evenly; then, under the condition that the ambient humidity is less than 45%, sequentially: tableting, coating, and subpackaging to obtain 1000 tablets of Grifola frondosa.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com