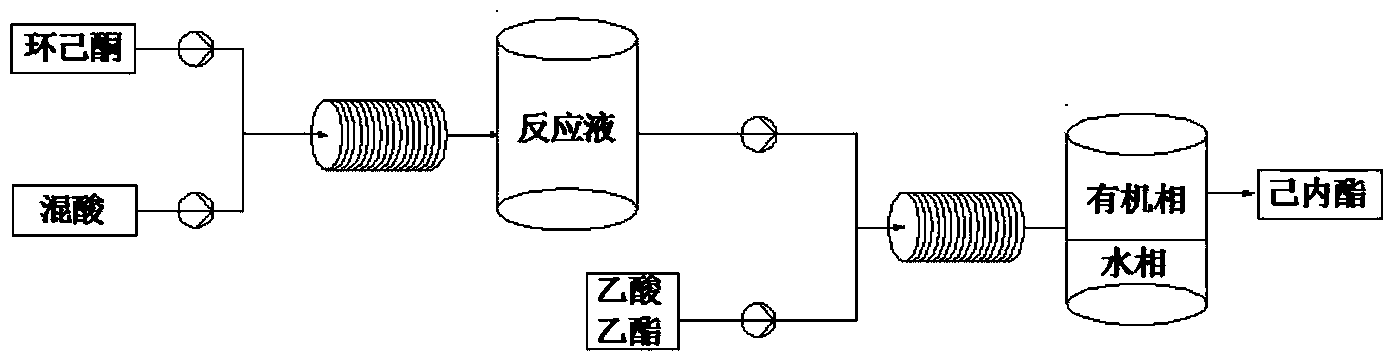
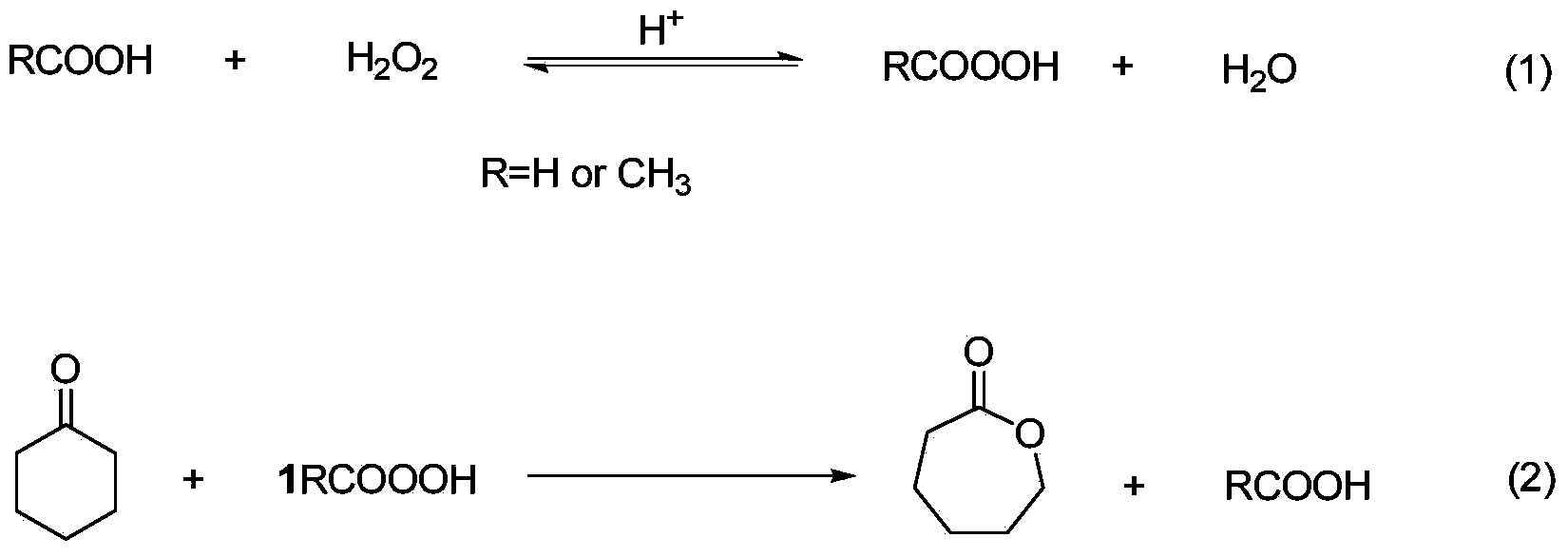
Method for preparing caprolactone through cyclohexanone catalyzed oxidation
A technology for catalytic oxidation and cyclohexanone, which is applied in the fields of chemical synthesis and technology, can solve the problems that caprolactone cannot accurately control the reaction temperature, cannot be continuously produced, and has low epoxidation speed, and achieves easy operation and control of the preparation process, The effect of short reaction residence time and improved conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The hydrogen peroxide of 30wt% and 98wt% acetic acid are mixed according to hydrogen peroxide: and the mol ratio of acetic acid is 1:1, adds catalyst concentrated H to wherein 2 SO 4 And stabilizer EDTA, the addition amount of catalyst is 6.31% of hydrogen peroxide quality, and the addition quality of stabilizer is 12.23% of hydrogen peroxide quality. The flow rate of cyclohexanone and the above mixture is controlled at 2.0ml / min and 4.6ml / min respectively, so that the molar ratio of acetic acid and cyclohexanone is 2.3:1, through the microchannel modular reaction device, the micro The reaction was carried out in a structural reactor, and the reaction retention time was 5.6 min. A length of polytetrafluorocapillary is connected to the back of the microchannel modular reaction device, and the polytetrafluorocapillary is immersed in an ice-water bath to terminate the reaction. The reactant is introduced into the separator, and 7wt% Na is added 2 CO 3 The aqueous solut...
Embodiment 2
[0036] 30wt% hydrogen peroxide and 98wt% acetic acid are mixed according to the molar ratio of hydrogen peroxide and acetic acid as 1:1, and catalyst concentrated H 3 PO 4 And stabilizer EDTA, the addition amount of catalyst is 4.32% of hydrogen peroxide quality, and the addition quality of stabilizer is 9.68% of hydrogen peroxide quality. The flow rate of cyclohexanone and the above mixture is controlled at 2.2ml / min and 4.4ml / min respectively, so that the molar ratio of acetic acid and cyclohexanone is 1.6:1, through the microchannel modular reaction device, the micro The reaction was carried out in a structural reactor, and the reaction retention time was 5.6 min. A length of polytetrafluorocapillary is connected to the back of the microchannel modular reaction device, and the polytetrafluorocapillary is immersed in an ice-water bath to terminate the reaction. The reactant is introduced into the separator, and 7wt% Na is added 2 CO 3 The aqueous solution was washed to a...
Embodiment 3
[0038] 30wt% hydrogen peroxide and 98wt% formic acid are mixed according to the molar ratio of hydrogen peroxide and formic acid as 1:1, and catalyst concentrated H 2 SO 4 And stabilizer EDTA, the addition amount of catalyst is 6.32% of hydrogen peroxide quality, and the addition quality of stabilizer is 12.23% of hydrogen peroxide quality. The flow rate of cyclohexanone and the above mixture is controlled at 2.0ml / min and 4.6ml / min respectively, so that the molar ratio of acetic acid and cyclohexanone is 2.3:1, through the microchannel modular reaction device, the micro The reaction was carried out in a structural reactor, and the reaction retention time was 5.6 min. A length of polytetrafluorocapillary is connected to the back of the microchannel modular reaction device, and the polytetrafluorocapillary is immersed in an ice-water bath to terminate the reaction. The reactant is introduced into the separator, and 7wt% Na is added 2 CO 3 The aqueous solution was washed to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com