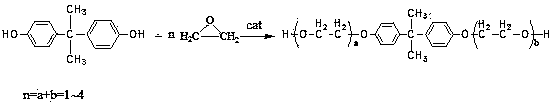
Method for preparing bis(hydroxyethyl) bisphenol A ether
A bis-hydroxyethyl bisphenol and a certain amount of technology are applied in the field of organic compound synthesis, and can solve the problems of easy isomerization of propylene oxide, weak reaction activity, long reaction period and the like, and achieve light product color and reaction period. Short, reduced by-product formation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
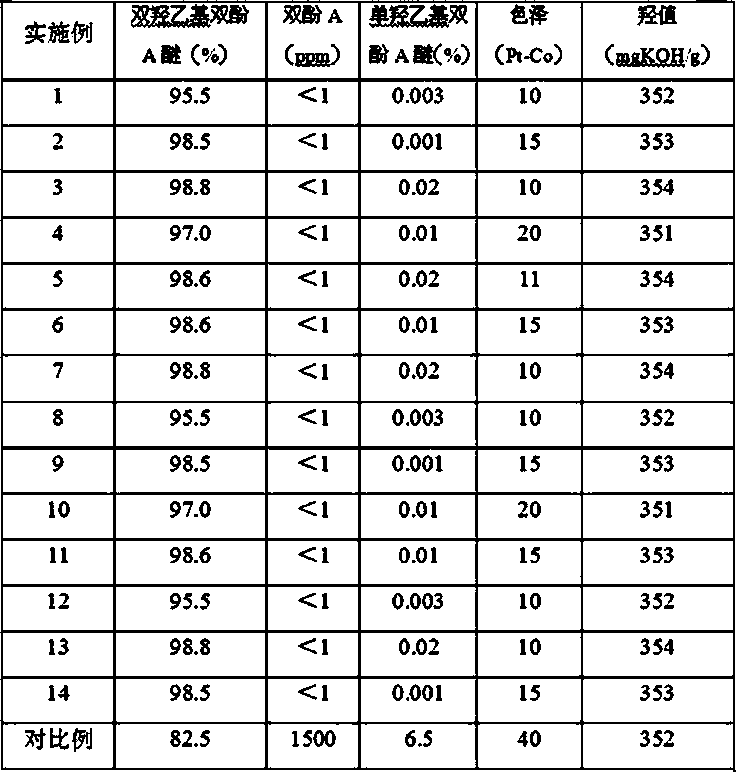
[0037] Add 700g of bisphenol A, 2.0g of composite catalyst (1.8g of 1,1'-bis(diphenylphosphine) ferrocene, 0.2g of solid NaOH) into the reaction kettle, vacuumize with a vacuum pump, and use N 2 After replacing the air in the reactor for three times, turn off the vacuum and start to heat up at a vacuum degree ≥ -0.096MPa. The temperature rises to 160°C, and after vacuum degassing for 10 minutes, continue to add 300g of ethylene oxide, control the reaction temperature at 165°C-170°C, and the pressure in the reactor at -0.04 MPa-0.3MPa. After the addition, keep warm and continue the reaction. until the pressure no longer drops. After the reaction is completed, the temperature is lowered to 100°C and vacuum degassed for 20 minutes, then neutralized by adding 0.2g of glacial acetic acid, then cooled to 80°C and discharged to obtain the finished product. The product was analyzed by liquid chromatography: the residual bisphenol A was <1ppm, the content of monohydroxyethyl bisphenol...
Embodiment 2
[0039] Add 700g of bisphenol A, 5.0g of composite catalyst (4.7g of 1,1'-bis(diphenylphosphine) ferrocene, 0.3g of solid potassium carbonate) into the reaction kettle, vacuumize with a vacuum pump, and use N 2 After replacing the air in the reactor for three times, turn off the vacuum and start to heat up at a vacuum degree ≥ -0.096MPa. The temperature rises to 160°C, and after vacuum degassing for 10 minutes, continue to add 278g of ethylene oxide, control the reaction temperature at 155°C-165°C, and the pressure in the reactor at -0.02 MPa-0.2MPa. After the addition, keep warm and continue the reaction. until the pressure no longer drops. After the reaction is completed, cool down to 100°C and vacuum degas for 20 minutes, then add 0.3g of glacial acetic acid for neutralization, then cool down to 80°C and discharge to obtain the finished product. The product was analyzed by liquid chromatography: the residual bisphenol A was <1ppm, the content of monohydroxyethyl bisphenol A...
Embodiment 3
[0041] Add 700g of bisphenol A, 1.5g of composite catalyst (1.35g of 1,1'-bis(diphenylphosphine)ferrocene, 0.15g of solid potassium methoxide) into the reaction kettle, vacuumize with a vacuum pump, and use N 2 After replacing the air in the reactor for three times, turn off the vacuum and start to heat up at a vacuum degree ≥ -0.096MPa. The temperature rises to 160°C, and after vacuum degassing for 10 minutes, continue to add 290g of ethylene oxide, control the reaction temperature at 160°C-165°C, and the pressure in the reactor at 0.0 MPa-0.4MPa. After the addition, keep warm and continue the reaction until until the pressure no longer drops. After the reaction is completed, the temperature is lowered to 100°C and vacuum degassed for 20 minutes, then neutralized by adding 0.15g of glacial acetic acid, then cooled to 80°C and discharged to obtain the finished product. The product was analyzed by liquid chromatography: the residual bisphenol A was <1ppm, the content of monohy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hydroxyl value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com