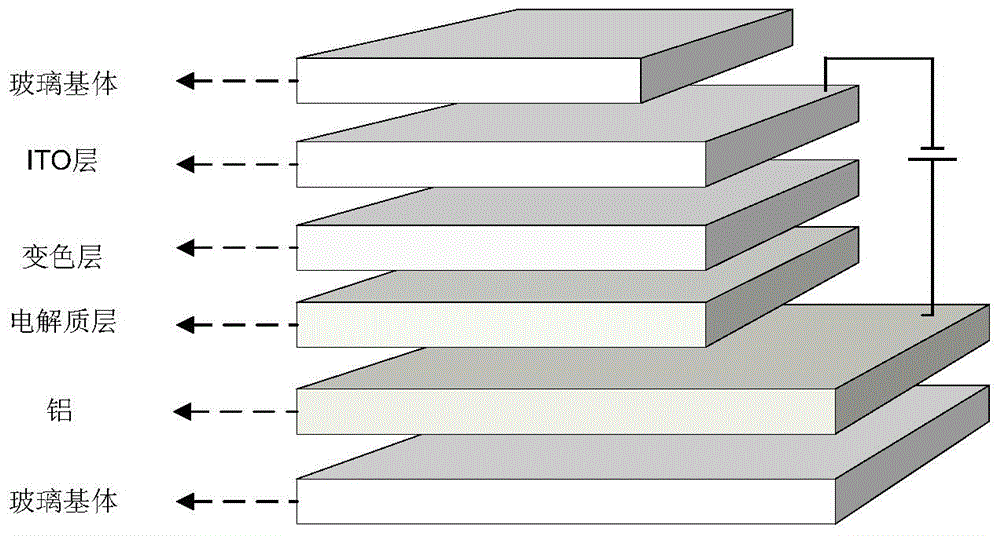
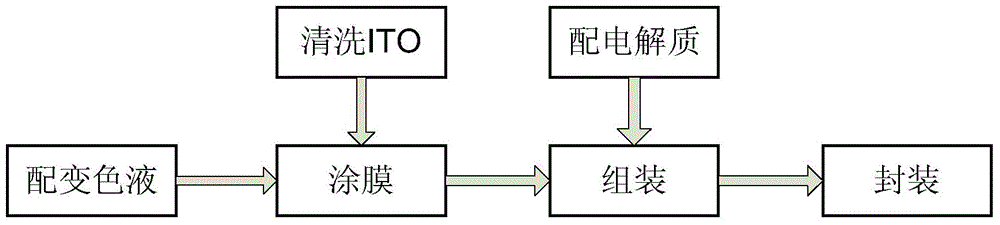
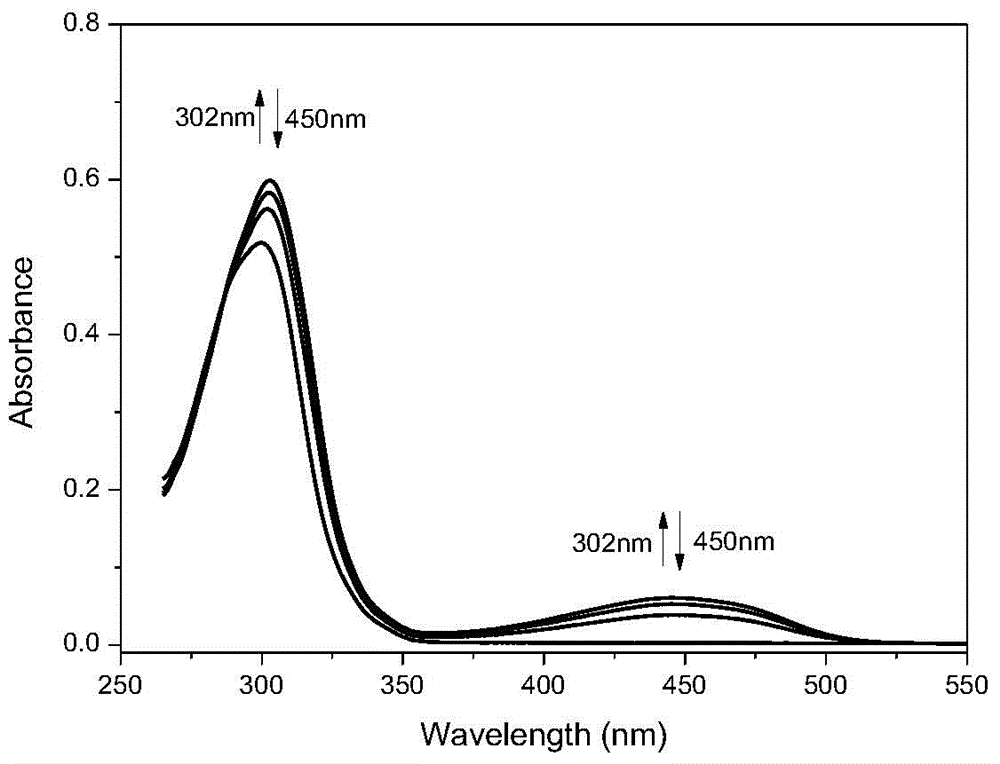
Photochromic and electrochromic bifunctional compound and preparation method thereof
An electrochromic and photochromic technology, applied in chemical instruments and methods, organic chemistry, color-changing fluorescent materials, etc., can solve the problem of high cost, inability to increase molecular switching states, and the lack of relative properties of photochromic and electrochromic properties. Independence and other issues to achieve the effect of capacity increase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~3
[0036] Examples 1-3 are 1,2-bis(3-(2,5-dimethylthienyl))-4-(4-(N,N-dianilinophenyl))cyclopentene (3) Synthesis
Embodiment 1
[0037] Example 1: Synthesis of 1-(3-(2,5-dimethylthienyl))-3-(4-(N,N-diphenylaminophenyl))propenone (1)
[0038] Add 3-acetyl-2,5-dimethylthiophene (1.54g, 10mmol), 4-(N,N-diphenylamino)benzaldehyde (10mmol) into ethanol (140mL), stir, and add 15mL 13M sodium hydroxide solution was reacted at room temperature for 48 hours, suction filtered, washed with water, and recrystallized with ethanol to obtain 3.63 g of compound (1) (yellow powder, yield 92%). m.p.143.0~144.5℃; MS(APCI):m / z calcd for C 27 h 23 NOS:410.16[M+H] + ;found: 410.35; 1 H NMR (400MHz, CDCl 3 )δ: (ppm) 7.67 (d, J = 15.6Hz, 1H), 7.48 (d, J = 8.5Hz, 2H), 7.32 (t, J = 7.7Hz, 4H), 7.22 ~ 7.09 (m, 7H) ,7.08~6.99(m,3H),2.72(s,3H),2.46(s,3H); 13 C NMR (100MHz, CDCl 3 )δ: (ppm) 186.6, 150.0, 146.9, 146.4, 143.4, 137.1, 135.2, 129.5, 129.5, 128.1, 126.0, 125.4, 124.0, 122.6, 121.8, 15.8, 15.0.
Embodiment 2
[0039] Example 2: 1,5-bis(3-(2,5-dimethylthienyl))-3-(4-(N,N-dianilinophenyl))-1,5-pentanedione (2) Synthesis
[0040] N 2 Under protection, add 3-acetyl-2,5-dimethylthiophene (5.5mmol) and 20mL dry tetrahydrofuran (THF) to a 50mL four-necked flask, cool down to -25°C, and dropwise add lithium diisopropylamide (3mL, 2M), complete, react at this temperature for 30min, dropwise add 15mL of THF solution of the corresponding compound (1) (5mmol), complete, keep warm for 30min, naturally rise to room temperature for 2h, add water to quench the reaction, and Adjust the pH to 5-6 with 1N hydrochloric acid, extract with dichloromethane, wash with water, dry over sodium sulfate, spin dry, and separate by column chromatography to obtain 1.88 g of a yellow solid with a yield of 67%. m.p.44-45℃. 1 H NMR (400MHz, CDCl 3 )δ: (ppm) 7.21 (t, J = 7.7Hz, 4H), 7.09 (d, J = 8.3Hz, 2H), 7.06-6.93 (m, 10H), 3.88 (p, J = 6.9Hz, 1H) ,3.15(ddd,J=42.2,16.1,7.0Hz,4H),2.58(s,6H),2.39(s,6H); 13 C NM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com