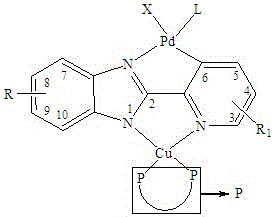
2-pyridyl benzimidazole palladium copper heteronuclear compound and its preparation method and application
A technology for pyridyl benzimidazoles and compounds is applied in the field of organic synthesis and achieves the effects of high yield, wide range of reaction substrates and mild catalytic reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Preparation of 2-pyridylbenzimidazole palladium copper heteronuclear compound (1)
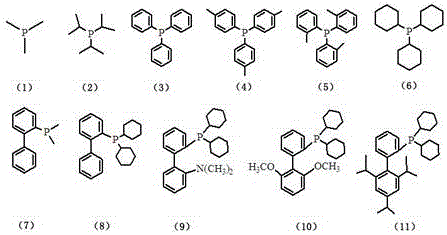
[0034] Add 2-pyridylbenzimidazolium copper bisphosphine compound A (1 mmol), Li 2 PdCl 4 (1 mmol), sodium acetate (1 mmol), 10 ml of anhydrous methanol, temperature 10 ° C, stirring the reaction solution for 12 hours and then filtering. After the obtained solid was dried, it was added to a 10ml flask, and then triphenylphosphine (1 mmol) and 5ml of dichloromethane were added in sequence. After stirring the reaction solution at 20°C for 1 hour, the solvent was distilled off, and dichloromethane and petroleum ether were used to Recrystallized from a mixed solvent to obtain product 1 with a yield of 86%. The obtained product was subjected to nuclear magnetic resonance analysis, and the data were as follows: 1H NMR: δ= 8.71 (d, 1H), 7.93 (d, 1H), 7.81 (d, 2H), 7.57-7.63 (m, 21H), 7.26-7.35 ( m, 17H), 1.83 (t, 4H).
[0035] The structural formula of the 2-pyridylbenzimidazole c...
Embodiment 2
[0037] Example 2: Preparation of 2-pyridylbenzimidazole palladium copper heteronuclear compound (3):
[0038] Add 2-pyridylbenzimidazolium copper bisphosphine compound B (1 mmol), Li 2 PdBr 4 (1.2mmol), sodium acetate (1.1 mmol), 12ml of anhydrous methanol, temperature 20°C, stirring the reaction solution for 20h and then filtering. After drying the obtained solid, add it to a 10ml flask, then add triphenylphosphine (1.2 mmol) and 5ml dichloromethane in sequence, stir the reaction solution at 30°C for 3h, distill off the solvent, and use dichloromethane and petroleum ether Recrystallized from a mixed solvent to obtain product 3 with a yield of 88%. The obtained product was subjected to nuclear magnetic resonance analysis, and the data were as follows: 1H NMR: δ= 8.70 (d, 1H), 7.91 (d, 1H), 7.78 (d, 2H), 7.53-7.61 (m, 21H), 7.24-7.33 ( m, 16H), 2.48 (s, 3H), 1.81 (t, 4H).
[0039] The structural formula of the 2-pyridylbenzimidazolium bisphosphine compound B is as follows: ...
Embodiment 3
[0041] Example 3: Preparation of 2-pyridylbenzimidazole palladium copper heteronuclear compound (6):
[0042] Add 2-pyridylbenzimidazolium bisphosphine compound C (1 mmol), Li 2 PdCl 4 (1.5 mmol), sodium acetate (1.5 mmol), 15 ml of anhydrous methanol, temperature 30 ° C, stirring the reaction solution for 30 h and then filtering. After drying the obtained solid, add it to a 10ml flask, then add trimethylphosphine (1.3 mmol) and 5ml acetone in sequence, stir the reaction solution at 15°C for 6h, distill off the solvent, and use a mixture of dichloromethane and petroleum ether Solvent recrystallization gave product 6 with a yield of 85%. The obtained product was subjected to nuclear magnetic resonance analysis, and the data were as follows: 1H NMR: δ= 8.75 (d, 1H), 7.87 (d, 1H), 7.73 (d, 2H), 7.50-7.59 (m, 12H), 7.20-7.34 ( m, 8H), 3.85 (s, 3H), 1.80 (t, 4H), 0.98(s, 9H).
[0043] The structural formula of the 2-pyridylbenzimidazole cuprous bisphosphine compound C is as fol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com