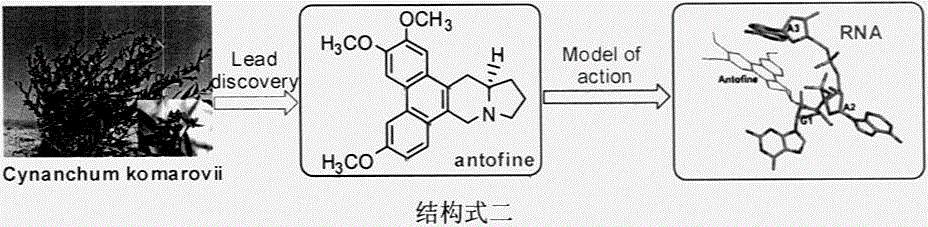
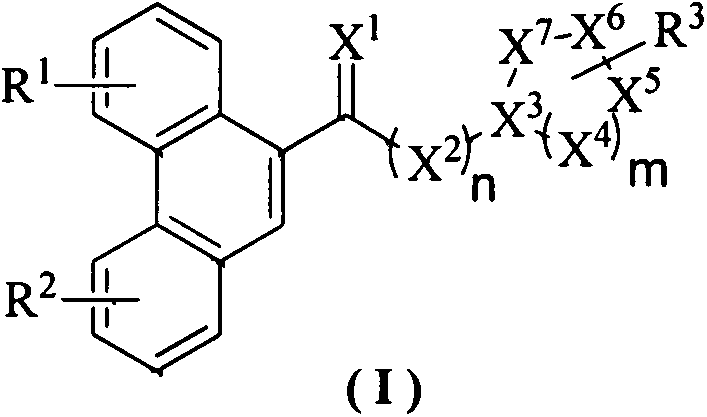
Phenanthrene-containing heterocyclic compounds, preparation method thereof and anti-plant virus application
An anti-plant virus agent and heterocyclic technology, applied in the field of pesticides, can solve the problems of high price of original drugs, easy resistance of viruses, and limited application range, and achieve significant anti-plant virus activity and excellent anti-plant virus activity , good environmental compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: Synthesis of phenanthrene-containing heterocyclic compound 1:
[0026]
[0027] Synthesis of 2,3,6,7-tetramethoxy-9-phenanthroyl chloride (37)
[0028] Add 0.80g (2.34mmol) of 2,3,6,7-tetramethoxy-9-phenanthroic acid (36) and 20mL oxalyl chloride into a 100mL single-necked bottle; add 2 drops of DMF in a water bath; then naturally rise to room temperature, After reacting for another 3 h, oxalyl chloride was evaporated under normal pressure to obtain 0.82 g of yellow solid 37; it was directly used in the next reaction without further treatment.
[0029] Synthesis of 1-(2,3,6,7-tetramethoxy-9-phenanthroyl)-3-methylpyrazole (1)
[0030] Add 0.23g 3-methylpyrazole, 25mL CH 2 Cl 2 , 0.34g Et 3 N, slowly dropwise add 0.82g of 2,3,6,7-tetramethoxy-9-phenanthroyl chloride (37) prepared above to CH 2 Cl 2 The solution was 20 mL, and after the dropwise addition was completed, it was slowly raised to room temperature and reacted for 2 hours, and TLC detected t...
Embodiment 2
[0031] Embodiment 2: Synthesis of phenanthrene-containing heterocyclic compound 19:
[0032]
[0033] Synthesis of 2,3,6,7-tetramethoxy-9-phenanthrenemethanol (38)
[0034] Add 1.03g of 2,3,6,7-tetramethoxy-9-phenanthroic acid (36) and 20mL THF into a 100mL single-necked bottle; add 0.28g of LiAlH in batches under cooling in an ice-water bath 4 , After the addition is complete, heat to reflux for 1.5h, then cool naturally to room temperature. Add 20 mL of CH to the reaction solution 2 Cl 2 , and then slowly add 1mol / L HCl dropwise in an ice-water bath until the white flocculent precipitate disappears; 2 Cl 2 (10mL×3) extraction; combined organic phase, washed once with saturated NaCl solution, anhydrous NaCl 2 SO 4 dry. Filtrate under reduced pressure and then concentrate to obtain a solid, which is then recrystallized with ethyl acetate to obtain a white solid with a yield of 95.0% and a melting point of 183-185°C. 1 H NMR (400MHz, CDCl 3 )δ7.83(s, 1H), 7.78(s, 1H...
Embodiment 3
[0039] Example 3: Synthesis of phenanthrene-containing heterocyclic compounds 2-18 and 20-35: by repeating the methods of Examples 1 and 2.
[0040] Synthesis of 1-(2,3,6,7-tetramethoxy-9-phenanthroyl)pyrazole (2)
[0041] Yellow solid, yield: 66.7%, melting point: 214-215°C. 1 H NMR (400MHz, CDCl 3 )δ8.47(d, J=2.8Hz, 1H), 7.99(s, 1H), 7.83-7.82(m, 2H), 7.80(s, 1H), 7.57(s, 1H), 7.26(s, 1H ), 6.59-6.58(m, 1H), 4.16(s, 3H), 4.14(s, 3H), 4.03(s, 3H), 3.95(s, 3H); 13 C NMR (100MHz, CDCl 3 I (m / z): calcd.for C 22 h 20 N 2 o 5 Na[M+Na] + 415.1264; found 415.1266.
[0042] Synthesis of 1-(2,3,6,7-tetramethoxy-9-phenanthroyl)imidazole (3)
[0043] Light yellow solid, yield: 79.0%, melting point: 209-210°C. 1 H NMR (400MHz, CDCl 3 )δ8.12(s, 1H), 7.84(s, 2H), 7.81(s, 1H), 7.66(s, 1H), 7.61(s, 1H), 7.24(s, 1H), 7.23(s, 1H ), 4.18(s, 3H), 4.16(s, 3H), 4.04(s, 3H), 3.98(s, 3H); 13 C NMR (100MHz, CDCl 3 I (m / z): calcd.for C 22 h 21 N 2 o 5 [M+H] + 393.1445; found 393....
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com