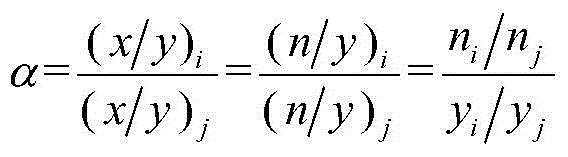Magnesium-based metal organic framework material for methane-nitrogen adsorption separation and preparation
A metal organic framework, nitrogen adsorption technology, applied in adsorption purification/separation, nitrogen purification/separation, separation methods, etc., can solve problems such as high cost and blockage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] (1) Weigh 36.9g MgSO 4 ·7H 2 O and 15ml (99% formic acid) were dissolved in 850ml of DMF, slowly added 2.8g of ammonium fluoride while stirring continuously, and stirred for 20min until uniform to obtain a mixed solution; the mixed solution was transferred to a 1L Teflon liner The autoclave was tightly sealed, placed in an oven at 120°C for 12 hours, and cooled down to room temperature naturally. The precipitate was separated by centrifugation (10000rpm, 5min), washed three times with 80ml of ethanol, and then dried at 100°C for 6 hours to obtain a white powder.
[0049] (2) The white powder was impregnated with 100 ml of acetone for 12 hours, filtered, and activated at 170° C. under a vacuum of 100 mbar for 12 hours to form adsorbent A, which weighed 14.3 g.
[0050] For comparative experiments, adsorbent B was prepared according to the method described in BASF patent US2011178335A1.
[0051] The specific surface area determined by the Langmuir method of gained adso...
Embodiment 2
[0058] (1) Weigh 49.3g MgSO 4 ·7H 2 O was dissolved in 720ml DMF to form solution A; 11.2g triethylenediamine was dissolved in 50ml deionized water to form solution B; solution B was slowly added to solution A while stirring continuously to form a uniform mixed solution. Add 82ml (98% methyl formate) dropwise to the mixed solution at a constant speed, stir for 20 minutes until uniform, and obtain mixed solution C; transfer the mixed solution to a 1L autoclave with a Teflon liner and seal it tightly, place it at 100 ℃ oven reaction for 24 hours, naturally cooled to room temperature. The precipitate was separated by centrifugation (10000rpm, 5min), washed three times with 80ml of methanol, and then dried at 100°C for 6 hours to obtain a white powder.
[0059] (2) The white powder was impregnated with 100 ml of acetone for 12 hours, filtered, and activated at 160° C. under a vacuum of 100 mbar for 12 hours to form adsorbent C, which weighed 16.1 g.
[0060] The specific surfac...
Embodiment 3
[0064] (1) Weigh 36.9g MgSO 4 ·7H 2O, 22.5g D-tartaric acid is dissolved in 607.6ml methanol (98%), slowly adds 2.8g ammonium fluoride while stirring constantly, stirs 30min until uniform, obtains mixed solution; Seal tightly in a ethylene-lined 1L autoclave, place in an oven at 120°C for 36 hours, and cool down to room temperature naturally. The precipitate was filtered out, washed three times with 300 ml of deionized water, and then the filter cake was dried at 60° C. for 10 hours to obtain a milky white lump.
[0065] (2) The product was activated at 130° C. for 6 hours under a vacuum of 100 mbar to form adsorbent D, which weighed 23.6 g.
[0066] The specific surface area determined by the Langmuir method of the obtained adsorbent D is 208m 2 / g, the average pore diameter is 2.4nm;
[0067] The obtained adsorbent D is at 298K, between 0-1MPa (absolute atmospheric pressure), αCH 4 / N 2 =2.6-4.4;
[0068] CH of the obtained adsorbent D 4 The dynamic adsorption capaci...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size distribution | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com