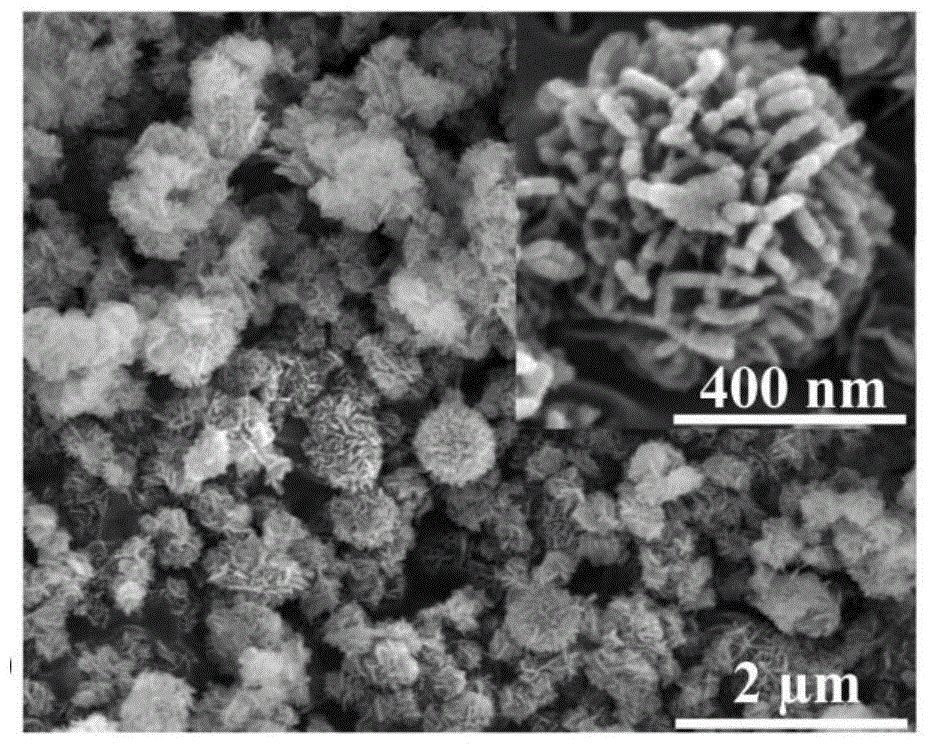
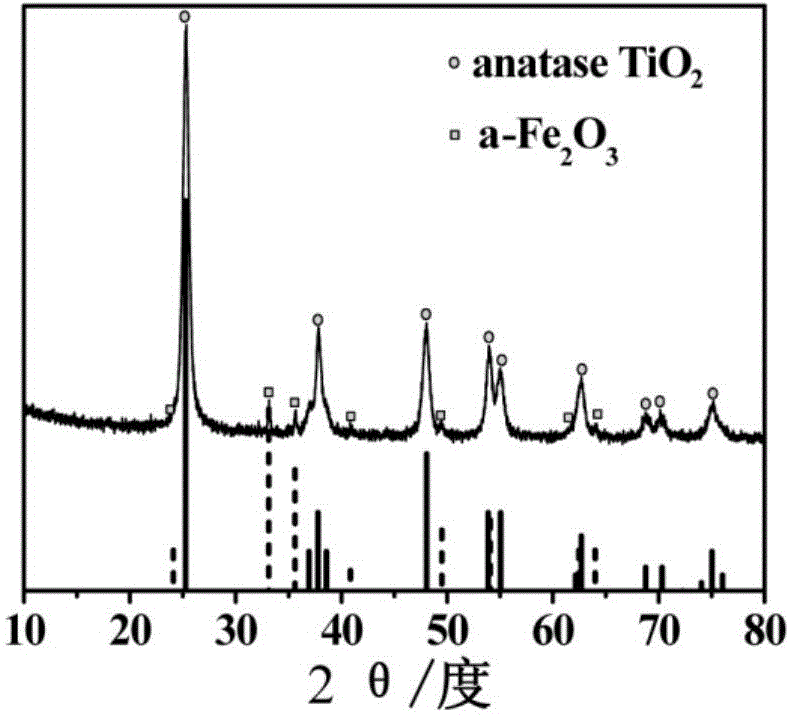
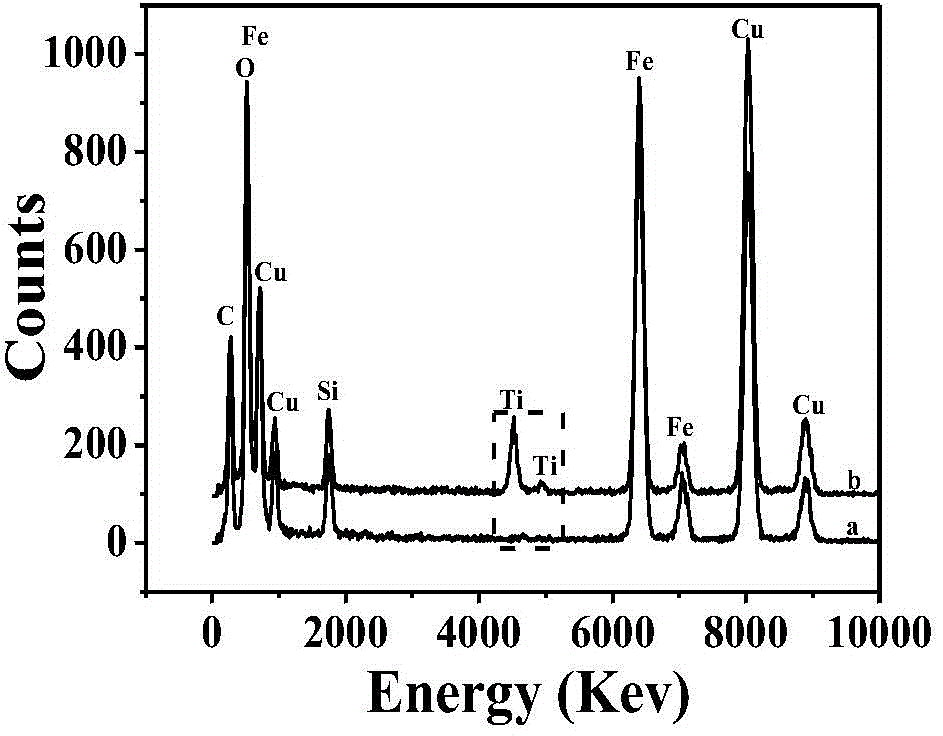
Hierarchical alpha-Fe2O3/TiO2 hollow sphere dual-functional photocatalyst and application thereof
A photocatalyst, -fe2o3 technology, applied in the direction of physical/chemical process catalyst, metal/metal oxide/metal hydroxide catalyst, alkali metal oxide/hydroxide, etc., can solve the problem of unfavorable recycling and reuse of powder , to achieve the effect of improving adsorption and photocatalytic oxidation/reduction performance, improving adsorption performance, and good adsorption performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Preparation of CuO: 2mmol of Cu(CH 3 COO) 2 ·H 2 The polyvinylpyrrolidone (PVP, molecular weight 58000) of O and 1mmol is dissolved in the dimethylformamide (DMF) of 25ml, the NaBH of 30mg 4 Add it to the above solution, stir until it is completely dissolved, heat the mixture to 80°C and keep it in a water bath for 15min, after the reaction is completed, the solid obtained is washed with ethanol three times to obtain Cu 2 O nanospheres, which were dried at 60°C, and then transferred to a muffle furnace for 30min at 300°C to obtain 0.153g of CuO monodisperse nanospheres;
[0034] (2) Preparation of iron oxyhydroxide: Add 50 mg of CuO nanospheres prepared above into 70 mL of deionized water, ultrasonically disperse for 10 minutes, and then add 50 mg of FeCl to the above solution 2 , keep the mixed solution in a polytetrafluoro autoclave at 170°C for 1h, after cooling to room temperature, use a mixed solution of ammonia water (mass concentration: 25-28%) and deioniz...
Embodiment 2
[0039] (1) Preparation of CuO: 2mmol of Cu(CH 3 COO) 2 ·H 2 The polyvinylpyrrolidone (PVP, molecular weight 58000) of O and 0.5mol is dissolved in the dimethylformamide (DMF) of 25ml, the NaBH of 30mg 4 Add to the above solution, stir until completely dissolved, heat the mixture to 80°C and keep it in a water bath for 15min, wash the product with ethanol 3 times to obtain Cu 2 O nanospheres, which were dried at 60°C, and then transferred to a muffle furnace for 30min at 300°C to obtain 0.155g of CuO monodisperse nanospheres;
[0040] (2) Preparation of iron oxyhydroxide: disperse 50 mg of CuO nanospheres prepared above into 70 mL of deionized water by sonication, and then add 50 mg of FeCl to the above solution 2, keep the mixed solution in a polytetrafluoro autoclave at 170°C for 1h, after cooling to room temperature, use a mixed solution of ammonia water (mass concentration: 25-28%) and deionized water (v / v=1 / 1) for precipitation Wash 3 times, wash with deionized water a...
Embodiment 3
[0045] (1) Preparation of CuO: 2mmol of Cu(CH 3 COO) 2 ·H 2 The polyvinylpyrrolidone (PVP, molecular weight 58000) of 0 and 2mmol is dissolved in the dimethylformamide (DMF) of 25ml, the NaBH of 30mg 4 Add to the above solution, stir until completely dissolved, heat the mixture to 80°C and keep it in a water bath for 15min, wash the product with ethanol 3 times to obtain Cu 2 O nanospheres, which were dried at 60°C, and then transferred to a muffle furnace for 30min at 300°C to obtain 0.155g of CuO monodisperse nanospheres;
[0046] (2) Preparation of iron oxyhydroxide: disperse 50 mg of CuO nanospheres prepared above into 70 mL of deionized water by sonication, and then add 50 mg of FeCl to the above solution 2 , keep the mixed solution in a polytetrafluoro autoclave at 170°C for 1h, after cooling to room temperature, use a mixed solution of ammonia water (mass concentration: 25-28%) and deionized water (v / v=1 / 1) for precipitation Wash 3 times, wash with deionized water a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com