Method for preparing cyclohexanol by catalyzing by base metal catalyst
A catalytic preparation and catalyst technology, applied in the direction of heterogeneous catalyst chemical elements, metal/metal oxide/metal hydroxide catalyst, hydrogenation preparation, etc., can solve the catalyst surface carbon activity, improve the production cost and life of cyclohexanone Shortening and other issues, to achieve good chemical stability, reduce synthesis costs, and easy separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
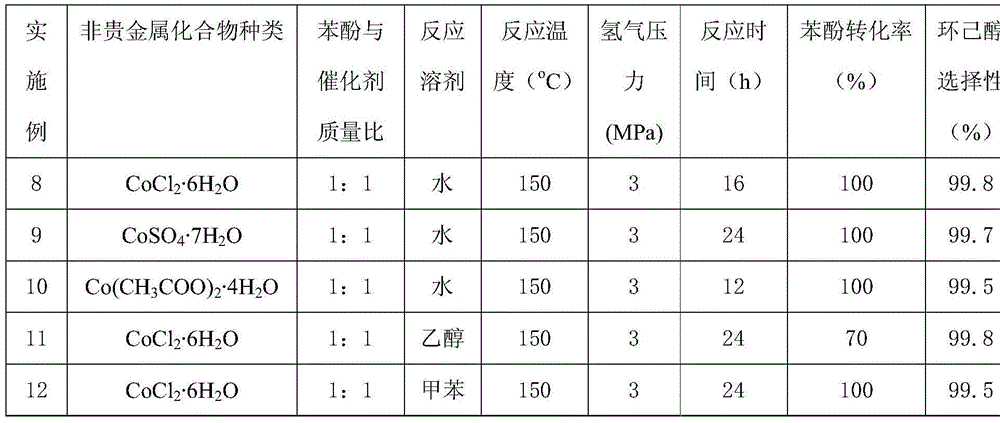
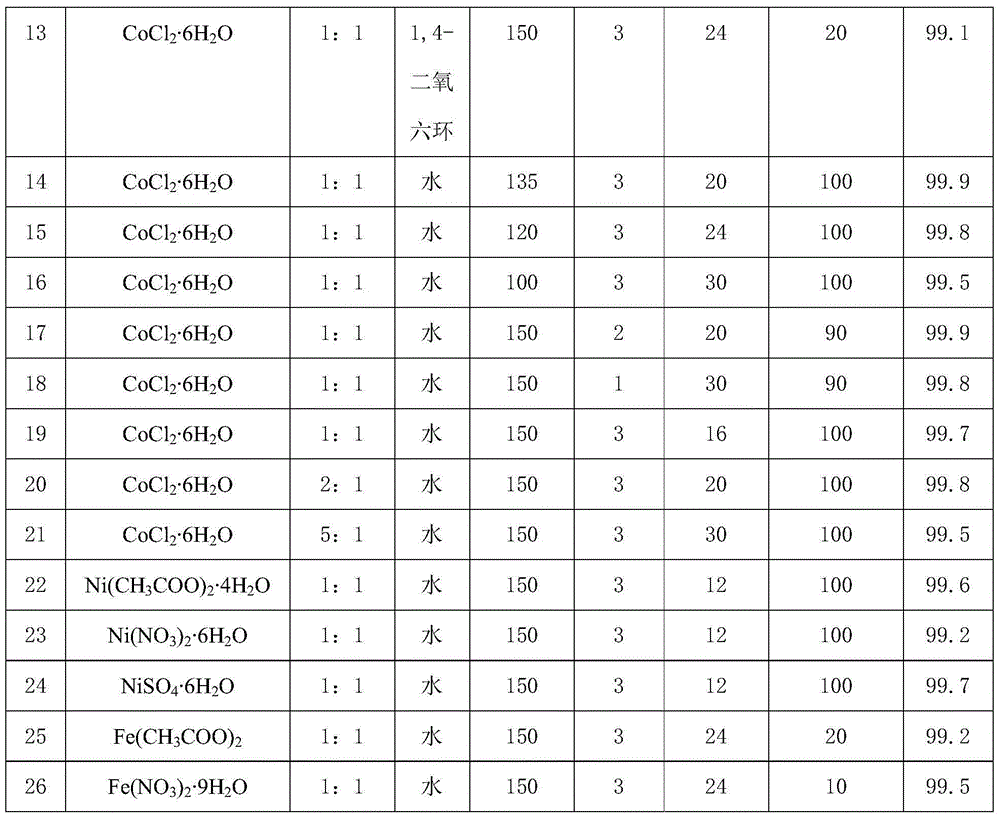
[0020] The preparation method of porous carbon material loaded base metal: with 1g glucosamine hydrochloride, 40g melamine and 0.85gCo(NO 3 ) 2 ·6H 2 O was dissolved in 300 mL of water, heated in an oil bath at 80°C, stirred by magnetic force, and evaporated to dryness completely. Then the obtained solid is fully ground and evenly calcined at a high temperature of 800°C to obtain a porous carbon material with a Co loading of about 25% and a nitrogen content of about 1.5%, namely 25CoCN 1.5 .
[0021] Phenol hydrogenation reaction: 100mg phenol, 10mL water, 100mg Co loaded porous carbon material 25CoCN 1.5 Add it into a 25mL autoclave, replace the atmosphere in the autoclave with hydrogen three times, fill the hydrogen pressure to 3MPa, seal the autoclave and raise the temperature to 150°C, stir it magnetically, and react for 20 hours. The catalyst is separated from the reaction liquid by means of filtration, the reaction liquid is detected by gas chromatography, and toluen...
Embodiment 2
[0023] The preparation method of porous carbon material loaded base metal: with 1g glucosamine hydrochloride, 40g melamine and 0.85gCo(NO 3 ) 2 ·6H 2 O was dissolved in 300 mL of water, heated in an oil bath at 80°C, stirred by magnetic force, and evaporated to dryness completely. Then the obtained solid is fully ground and evenly calcined at a high temperature of 900°C to obtain a porous carbon material with a Co loading of about 25% and a nitrogen content of about 2%, namely 25CoCN 2 .
[0024] Phenol hydrogenation reaction: 100mg phenol, 10mL water, 100mg Co loaded porous carbon material 25CoCN 2 Add it into a 25mL autoclave, replace the atmosphere in the autoclave with hydrogen three times, fill the hydrogen pressure to 3MPa, seal the autoclave and raise the temperature to 150°C, stir it magnetically, and react for 24 hours. The catalyst is separated from the reaction liquid by means of filtration, the reaction liquid is detected by gas chromatography, and toluene is u...
Embodiment 3
[0026] The preparation method of porous carbon material loaded base metal: with 1g glucosamine hydrochloride, 40g melamine and 0.85gCo(NO 3 ) 2 ·6H 2 O was dissolved in 300 mL of water, heated in an oil bath at 80°C, stirred by magnetic force, and evaporated to dryness completely. Then the obtained solid is fully ground and evenly calcined at a high temperature of 1000°C to obtain a porous carbon material with a Co loading of about 30% and a nitrogen content of about 1.5%, that is, 30CoCN 1.5 .
[0027] Phenol hydrogenation reaction: 100mg phenol, 10mL water, 100mg Co loaded porous carbon material 30CoCN 1.5 Add it into a 25mL autoclave, replace the atmosphere in the autoclave with hydrogen three times, fill the hydrogen pressure to 3MPa, seal the autoclave and raise the temperature to 150°C, stir it magnetically, and react for 30 hours. The catalyst is separated from the reaction liquid by means of filtration, the reaction liquid is detected by gas chromatography, and tol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com