Synthesis method of ethylidene diacetate from methyl acetate
A technology of diethylene acetate and its synthesis method, which is applied in the direction of carbon monoxide or formate reaction preparation, organic chemistry, etc., can solve the problems of low selectivity of diacetate and low conversion rate of methyl acetate, etc., and achieve The effect of increasing the conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
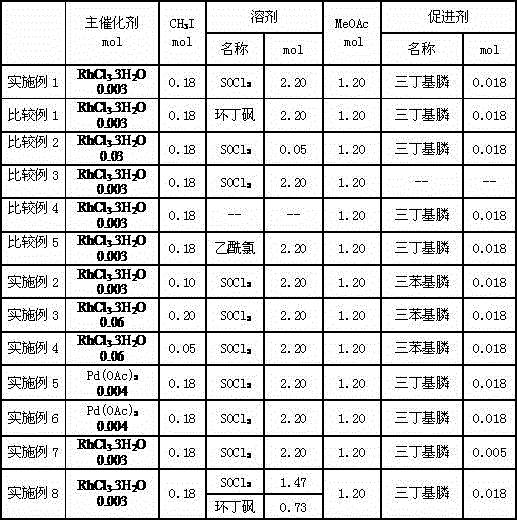
[0013] Synthesis of diethylene acetate: 2.20mol SOCl 2 , 0.003molRhCl 3 .3H 2 O, 0.18molCH 3 1, 0.018mol tributylphosphine and 1.20mol MeOAc add in the 2 liters of titanium reactors that have replaced the air wherein with argon in advance, pass into carbon monoxide and hydrogen then, make solvent: MeOAc:CO:H 2 =1:0.55:0.55:0.28, after reacting at 165° C. for 8 hours under stirring, stop the reaction.
[0014] Product analysis: The reaction mixture obtained from the above reaction was cooled, decompressed, and separated, and the liquid phase was analyzed by chromatography and nuclear magnetic resonance (GLC / NMR).
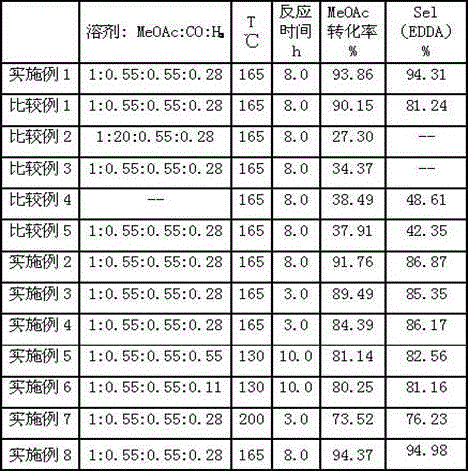
[0015] The transformation rate of methyl acetate reaches 93.86% through calculation, and the selectivity of diacetate ethylene is 94.31%. The yields and selectivities of ethyl esters are listed in Table 1.
Embodiment 2
[0037] 2.20mol SOCl 2 , 0.003molRhCl 3 .3H 2 O, 0.10molCH 3 1, 0.018mol triphenylphosphine and 1.20mol MeOAc add in the 2 liters of titanium reactors that have replaced the air wherein with argon in advance, pass into carbon monoxide and hydrogen then, make solvent: MeOAc:CO:H 2 =1:0.55:0.55:0.28, after reacting at 165° C. for 8 hours under stirring, stop the reaction.
[0038] Reaction conditions, each material feed amount, product after analysis and calculation, the conversion rate of methyl acetate and the selectivity and yield of diacetate are listed in Table 1.
Embodiment 3
[0040] 2.20mol SOCl 2 , 0.006molRhCl 3 .3H 2 O, 0.20molCH 3 1, 0.018mol triphenylphosphine and 1.20mol MeOAc add in the 2 liters of titanium reactors that have replaced the air wherein with argon in advance, pass into carbon monoxide and hydrogen then, make solvent: MeOAc:CO:H 2 =1:0.55:0.55:0.28, after reacting at 165° C. for 3 hours under stirring, stop the reaction.
[0041] Reaction conditions, each material feed amount, product after analysis and calculation, the conversion rate of methyl acetate and the selectivity and yield of diacetate are listed in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com