Impurity of erlotinib hydrochloride as well as preparation method and detection method thereof
A technology of erlotinib hydrochloride and impurities, which is applied to the process impurities of erlotinib hydrochloride and its preparation method and detection method, which can solve the problems affecting the quality of erlotinib hydrochloride products and adverse reactions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
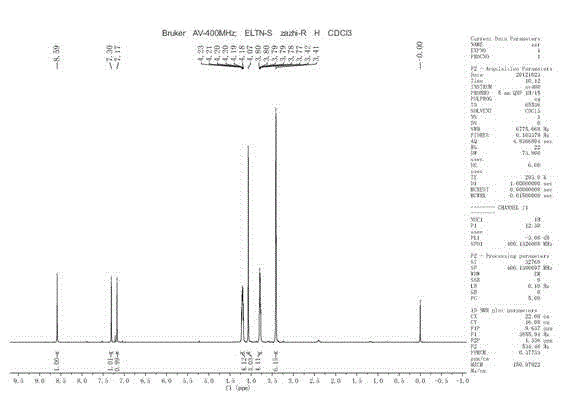
[0029] Example 1 Preparation of 4-methoxy-6,7-bis(2-methoxyethoxy)-quinazoline (Ⅰa)
[0030] Dissolve 1.0 g of 4-chloro-6,7-bis(2-methoxyethoxy)-quinazoline in 10 mL of methanol, and add 0.2 g of sodium methylate to the reaction solution in batches under stirring. After the addition, the stirring reaction was continued for 5 h, and the reaction was detected by TLC. After the reaction, the reaction solution was concentrated, the residue was dissolved in dichloromethane, washed with water and saturated brine in turn, the organic layer was dried, concentrated, the residue was slurried with ether, and filtered to obtain about 0.7 g of a white solid, yield: 71%. HRMS-ESI (m / z): 309.1445 (M +H + ); 1 H-NMR (400MHz, CDCl 3 ) δ = 8.59 (s, 1H), 7.30 (s, 1H), 7.17 (s, 1H), 4.23~4.18 (m, 4H), 4.07 (s, 3H), 3.80~3.77 (m, 4H), 3.42 (s , 3H), 3.41 (s, 3H) ppm.
Embodiment 2
[0031] Example 2 Preparation of 4-methoxy-6,7-bis(2-methoxyethoxy)-quinazoline (Ⅰa)
[0032] Under ice-bath condition, dissolve 0.6g NaH in 10mL dry tetrahydrofuran, under stirring condition, dissolve 2.0 g 6,7-bis(2-methoxyethoxy)-4-quinazolinone (compound of formula III) The tetrahydrofuran solution was slowly dropped into the reaction solution, and after the drop was completed, it was raised to room temperature and stirred for 1 hour. Subsequently, 1.2 g of methyl iodide was slowly dropped into the reaction liquid, and stirred for 3 hours. After the reaction, the reaction solution was concentrated, and the residue was purified by column chromatography (eluent: ethyl acetate:petroleum ether = 1:1) to obtain about 0.8 g of a white solid with a yield of 41%.
Embodiment 3
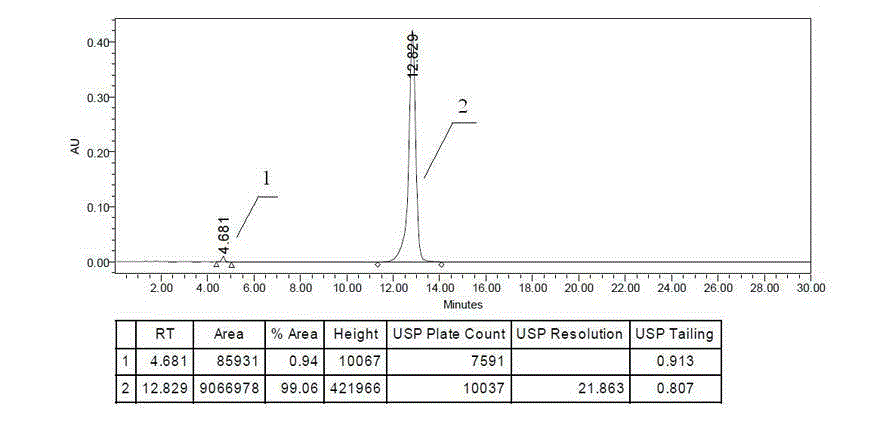
[0033] Example 3 Application of Etherified Impurity Ⅰa of Erlotinib Hydrochloride as Impurity Control in High Performance Liquid Chromatography
[0034] It is used for the analysis of the impurity involved in the analysis of erlotinib hydrochloride bulk drug and its preparations. Chromatographic column used in high performance liquid chromatography: venusil XBP C8 4.6×150mm 5um, column temperature: 30°C, flow rate: 1.0ml / min, wavelength: 247nm, mobile phase: acetonitrile-0.05mol / L potassium dihydrogen phosphate solution (take Dissolve 6.80g of potassium dihydrogen phosphate in 1000ml of water, adjust the pH to 7.0 with phosphoric acid)=58:42.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com