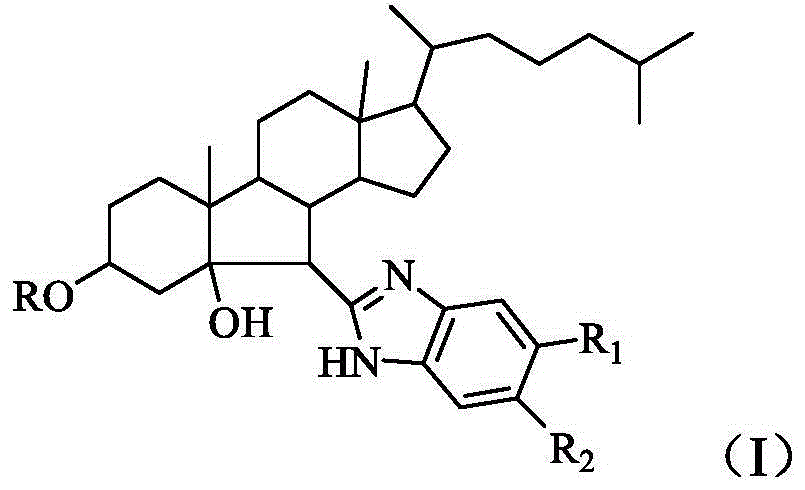
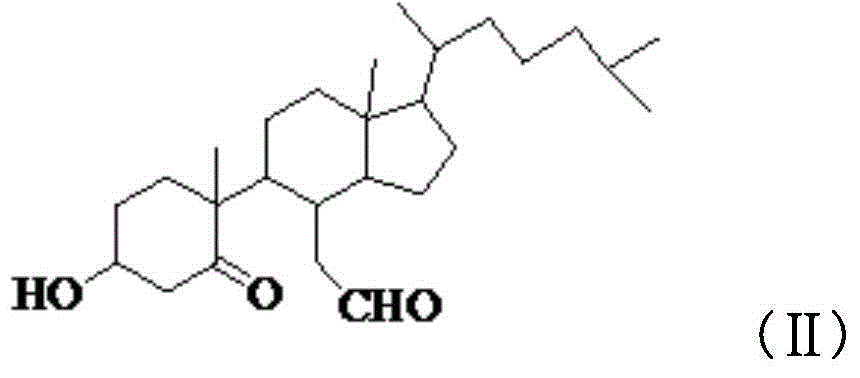
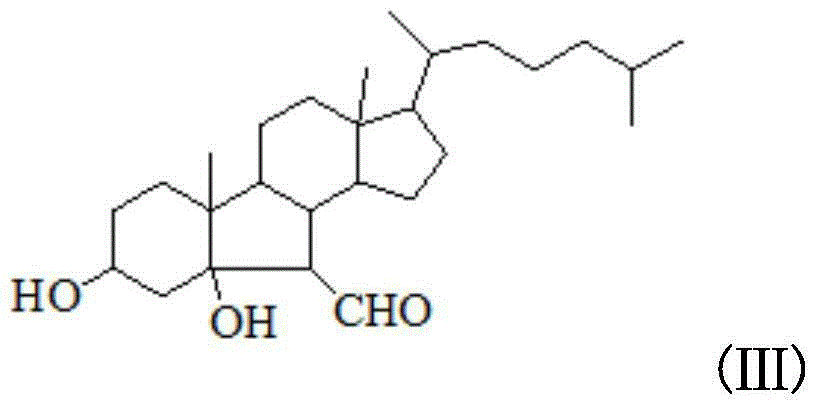
B-norcholestane benzimidazole compound as well as preparation method and application thereof
A benzimidazole and compound technology, applied in the field of compound preparation, can solve the problems of high toxicity and limited use range, and achieve the effects of low damage, simple preparation method, and increased inhibition rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Preparation of 2-(B-nor-3',5'-dihydroxy-6'-cholestanyl)-5-fluorobenzimidazole.
[0055] Step 1. Put 400mg of cholesterol in a large test tube, add 20mL of dichloromethane and 5mL of methanol, mix, place in a liquid nitrogen-cooled Dewar flask, cool to -60°C, and pass through an ozone-rich Oxygen flowed until the reaction was completed, and ozone was stopped after 20 minutes. At this time, the solution turned light blue, and then 1 mL of dimethyl sulfide was added, and the temperature was raised to 20 ° C. Stirring, the solvent was removed from the stirred substance to obtain a colorless The oily substance is the first compound;
[0056] Step 2. Put 400mg of the first compound in a 100mL round bottom flask, add 20mL of benzene and 2g of neutral alumina, mix and stir at 25°C, and separate the reacted material on a silica gel column. The substance after removing the solvent is purified to obtain the second compound;
[0057] Step 3. Put 350mg of the second compound in a ...
Embodiment 2
[0064] Preparation of 2-(B-nor-3',5'-dihydroxy-6'-cholestanyl)-5-nitrobenzimidazole.
[0065] Step 1. Put 600mg of cholesterol in a large test tube, add 60mL of dichloromethane and 15mL of methanol, mix, place in a liquid nitrogen-cooled Dewar flask, cool to -90°C, and pass through an ozone-rich Oxygen flowed until the reaction was completed, and ozone was stopped after 40 minutes. At this time, the solution turned light blue, and then 8 mL of dimethyl sulfide was added, heated to 35 ° C, stirred, and the solvent was removed from the stirred substance to obtain a colorless The oily substance is the first compound.
[0066] Step 2. Put 600mg of the first compound in a 100mL round bottom flask, add 40mL of benzene and 7g of neutral alumina, mix and stir at 25°C, and separate the reacted material on a silica gel column. The resulting substance was removed from the solvent and purified to obtain the second compound.
[0067] Step 3. Put 500mg of the second compound in a 100mL ro...
Embodiment 3
[0074] Preparation of 2-(B-nor-3',5'-dihydroxy-6'-cholestanyl)-5-trifluoromethylbenzimidazole.
[0075] Step 1. Put 500mg of cholesterol in a large test tube, add 40mL of dichloromethane and 10mL of methanol, mix, place in a liquid nitrogen-cooled Dewar flask, cool to -78°C, and pass through an ozone-rich Oxygen flowed until the reaction was completed, and ozone was stopped after 30 minutes. At this time, the solution turned light blue, and then 4 mL of dimethyl sulfide was added, heated to 25 ° C, stirred, and the solvent was removed from the stirred substance to obtain a colorless The oily substance is the first compound.
[0076] Step 2. Put 566mg of the first compound in a 100mL round bottom flask, add 30mL of benzene and 5g of neutral alumina, mix and stir at 25°C, and separate the reacted material on a silica gel column. The resulting substance was removed from the solvent and purified to obtain the second compound.
[0077] Step 3: Put 327mg of the second compound in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com