Polypeptide for targeting combination with PSMA extracellular domain and application of polypeptide
A targeted, extramembranous region technology, applied in the medical field, can solve the problems of poor prognosis and high mortality of PCa, and achieve good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1. Synthesis and quality control of polypeptide DOTA-PTP
[0033] 1. Synthesis of linear polypeptide PTP by solid-phase method and coupling with 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA)
[0034] ① Polypeptides are synthesized from the C-terminus to the N-terminus. First, hang the first amino acid on the resin, weigh 2.0g of the resin into a clean and dry reaction tube, add an appropriate amount of dimethylformamide (DMF), activate it for about 30 minutes, and then Weigh the first amino acid Fmoc-Ser(But)-OH 0.5mmol, 4-dimethylaminopyridine (DMAP) 75mg, N,N'-diisopropylcarbodiimide (DIC) 1ml into the reaction tube , DMF as a solvent reaction for 3h. After the reaction, wash with DMF for 4 to 6 times, add appropriate amount of pyridine and methanol, the volume ratio is 1:1, and react for 30 minutes. After the reaction, wash with DMF 4 to 6 times. Then use piperidine solution to remove the Fmoc of amino acid, remove twice for a total of 15min, 10m...
Embodiment 2
[0056] Example 2: 68 Preparation of Ga-DOTA-PTP and its in vivo and in vitro experiments
[0057] one, 68 Preparation of Ga-DOTA-PTP
[0058] Configure DOTA-PTP polypeptide solution as 2mg / ml, HEPES as 1M (pH: 7), 68 GaCl 3 1-10mCi / ml.
[0059] Take 25 μl of HEPES solution (a non-ionic amphoteric buffer, composed of 4-hydroxyethylpiperazineethanesulfonic acid, distilled water and sodium hydroxide) (concentration 1M), and add 0.7-5 μl DOTA-PTP polypeptide solution in sequence ( 2mg / ml) and 100μl of 68 GaCl 3 (radioactive concentration 1-10mCi / ml), placed in a water bath at 95°C and heated for 15-20min to obtain 68 Ga-DOTA-PTP.
[0060] two, 68 Ga-DOTA-PTP in vitro experiment
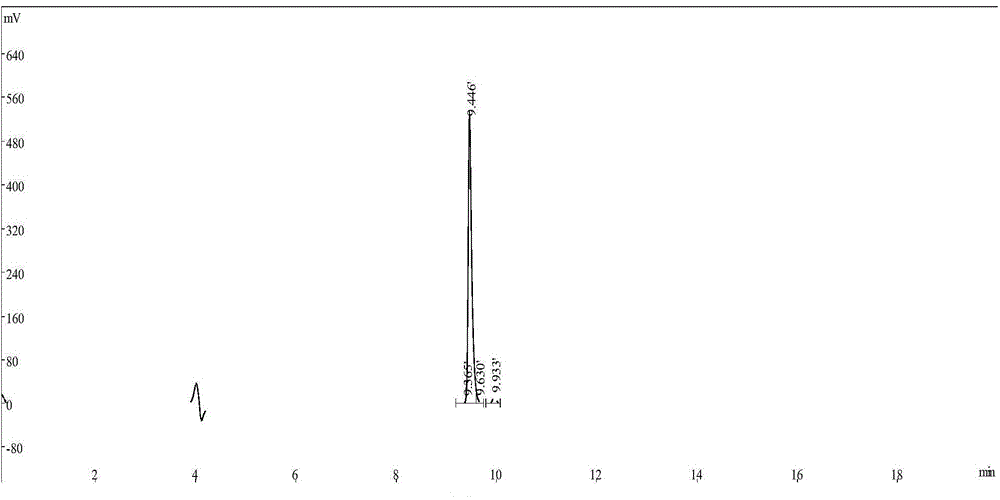
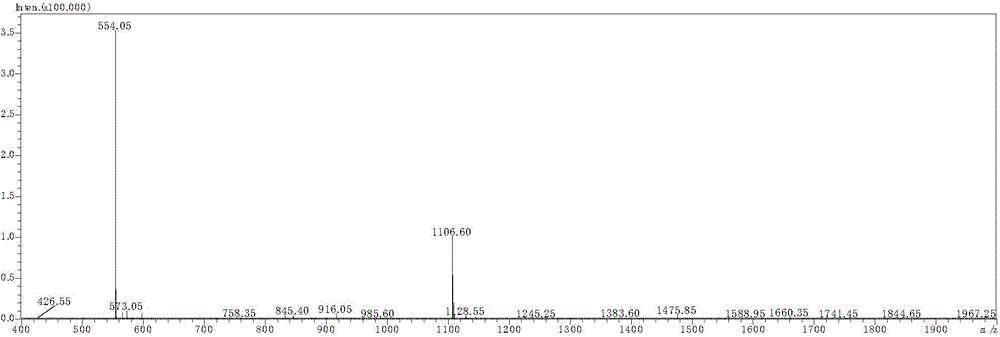
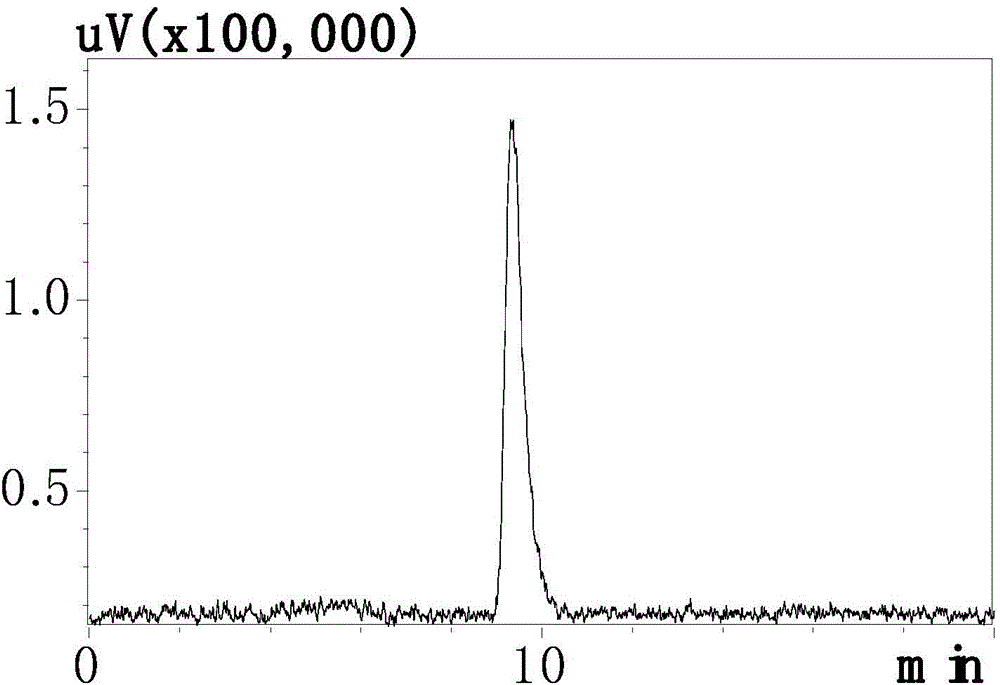
[0061] 68 After Ga-DOTA-PTP was synthesized, it was detected by radioactive-HPLC. The method and parameters were the same as before, and the results showed that 68 The HPLC peak of Ga-DOTA-PTP and the radioactive peak appear at the same time, see image 3 and 4 , radiochemically pure (98.4 ±...
Embodiment 3
[0072] Example 3: 64 Preparation of Cu-DOTA-PTP and its in vivo and in vitro experiments
[0073] In addition to radionuclides for 64 CuCl 2 Outside, all the other are with embodiment 2.
[0074] Result 1: 64 The radiochemical purity of Cu-DOTA-PTP was (98.3±1.31%), and it was incubated in normal saline at 37°C. After 2 hours, 4 hours, and 6 hours, 20ul were taken for HPLC inspection, and the results were not found. 64 The Cu free peak indicates that it has good stability in vitro, and its radiochemical purity is (96.5±0.76%) at 6 hours.
[0075] Result 2: Prostate cancer tumor-bearing mice 64Cu-DOTA-PTP after tail vein injection, the biological distribution in vivo and 68 Ga-DOTA-PTP is similar, and the main reason is that the biological distribution characteristics in the body mainly depend on DOTA-PTP, which is mainly excreted through the liver and kidney, and cleared quickly. After 60 minutes, the radioactivity uptake in the liver and kidneys can be significantly red...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com