Two-dimensional ultrathin tin sulfide nanosheets, and preparation method and application thereof
A nanosheet, tin sulfide technology, applied in chemical instruments and methods, nanotechnology, nanotechnology, etc., to achieve the effect of good cycle performance, simple process and high specific capacitance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
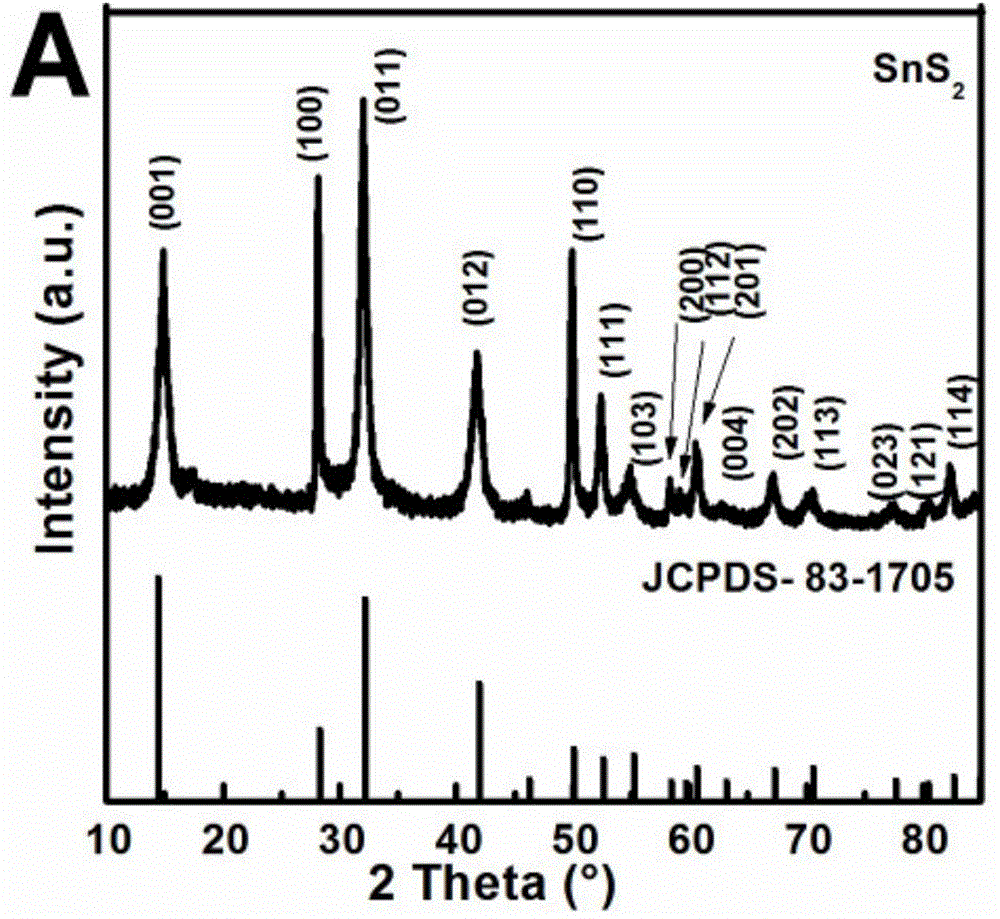
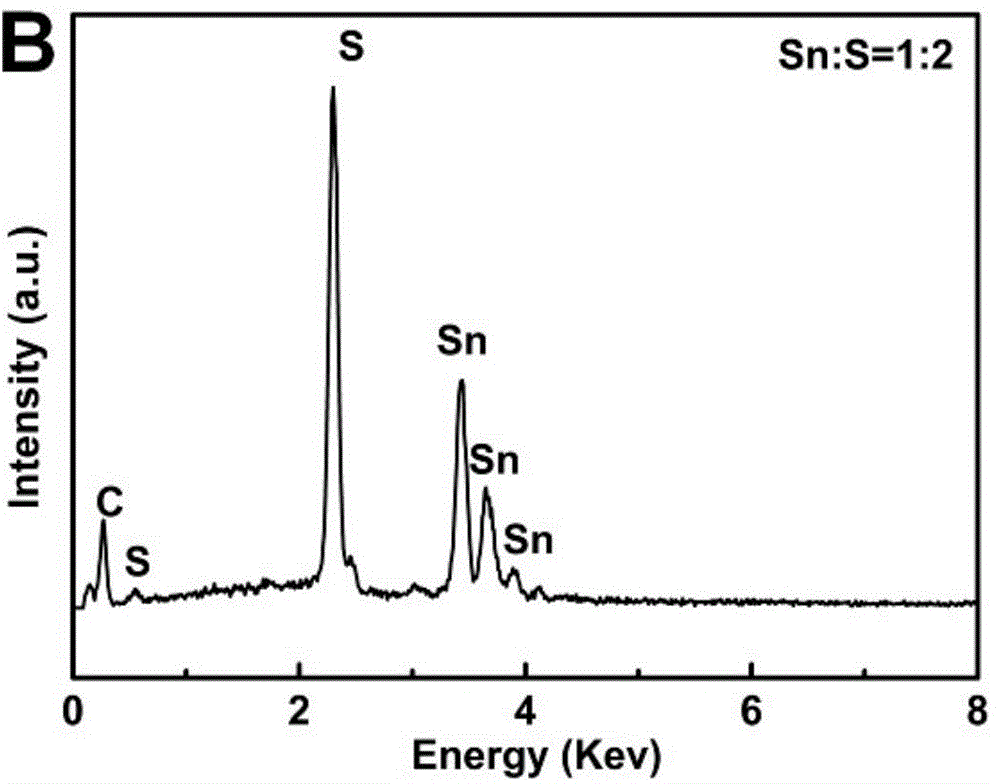
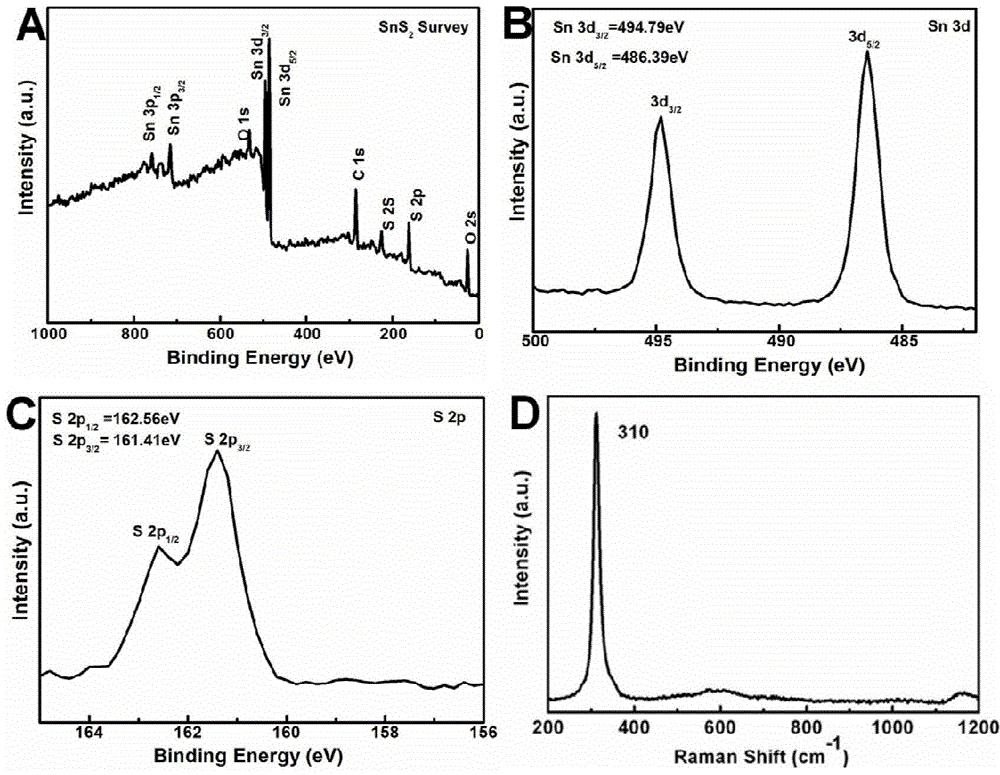
[0034] At room temperature, in a clean and dry 100mL three-necked flask, add 0.25mmol Sn 2 Cl 4 (Tu) 5 2H 2 O and 10mL oleic acid, ultrasonic 5min to obtain a suspension. Heat the mixture in a sand bath at 5 °C min -1 The heating rate is increased from room temperature to 180-230 ° C, and the heating is stopped after 30 minutes of heat preservation. During the heating process, it can be seen that before 100°C, the color of the suspension does not change substantially and is colorless. Subsequently, bubbles gradually emerged, and the solid matter began to decompose, and the color gradually changed from colorless to light yellow, then brown, and finally dark brown. After the reaction, the reaction product was dispersed and settled with absolute ethanol, centrifuged, washed with ethanol and n-hexane, and dried by centrifugation. The final target product was vacuum-dried at room temperature for 3-5 hours for subsequent analysis and characterization.
[0035] The products wer...
Embodiment 2
[0037] At room temperature, in a clean and dry 100mL three-necked flask, add 0.25mmol Sn 2 Cl 4 (Tu) 5 2H 2 O and 10mL oleic acid, ultrasonic 5min to obtain a suspension. Heat the mixture in a sand bath at 5 °C min -1 The heating rate is increased from room temperature to 300-330 ° C, and the heating is stopped after 30 minutes of heat preservation. After the reaction, the product was naturally cooled to room temperature and centrifuged, washed with n-heptane and absolute ethanol several times, and dried in a vacuum oven for subsequent analysis and characterization.
[0038] Same as in Example 1, the target product SnS nanocrystals were characterized by X-Ray energy spectrometer (EDX), element surface distribution map (Mapping), XRD, TEM and HRTEM, etc. XRD results see Figure 5 .
Embodiment 3
[0040] At room temperature, in a clean and dry 100mL three-necked flask, add 0.25mmol Sn 2 Cl 4 (Tu) 5 2H 2 O and 10mL oleic acid, ultrasonic 5min to obtain a suspension. Heat the mixture in a sand bath at 5 °C min -1 The heating rate is increased from room temperature to 260-295 ° C, and the heating is stopped after 30 minutes of heat preservation. After the reaction, the product was naturally cooled to room temperature and centrifuged, washed with n-heptane and absolute ethanol several times, and dried in a vacuum oven for subsequent analysis and characterization.
[0041] Same as in Example 1, the target product SnS nanocrystals were characterized by X-Ray energy spectrometer (EDX), element surface distribution map (Mapping), XRD, TEM and HRTEM, etc. XRD results see Figure 5 .
[0042] According to Examples 1-3, the method of the present invention adopts solid-liquid phase chemical reaction to synthesize tin sulfide nanocrystals, and the hexagonal phase SnS can be o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| capacitance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com