Synthesis method and application of polyhydroxy stearate
A technology of polyhydroxystearate and polyhydroxystearic acid, applied in the direction of carboxylate preparation, carboxylate preparation, chemical instruments and methods, etc., can solve the complex post-treatment of 9,10-dihydroxystearic acid , non-environmental protection, strong corrosion and other problems, to save formula space, increase zinc content, reduce corrosion and toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: the preparation method of dihydroxystearic acid
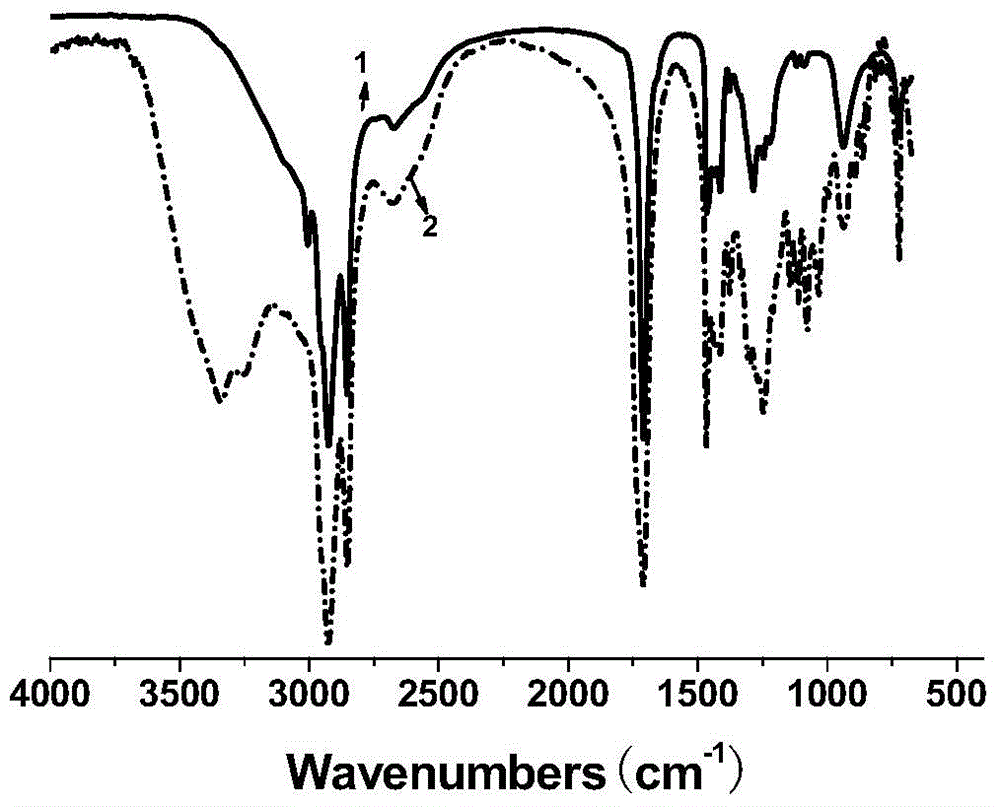
[0031] Add 11.3g of oleic acid, 16.0g of industrial acetic acid (50% by mass fraction) and 4.0g of hydrochloric acid with 40% by mass fraction in a three-necked flask with stirring and a thermometer, and slowly dropwise add 30% by mass fraction to the system at room temperature 12.0 g of hydrogen peroxide was heated up to 55° C., and reacted for 6 hours under vigorous stirring. After the reaction was completed, it was suction filtered, washed with hot water for 3 times, and then recrystallized with ethyl acetate to obtain a white powdery solid which was dihydroxystearic acid, with a melting point of 92-96° C. and a yield of 97%. Take the product for infrared spectroscopy detection, such as figure 1 As shown, the result is displayed at 3400cm -1 There is a relatively obvious peak at , which is attributed to the hydroxyl functional group in the molecular structure, so it can be judged that the hydroxyl func...
Embodiment 2
[0032] Embodiment 2: the preparation method of tetrahydroxystearic acid
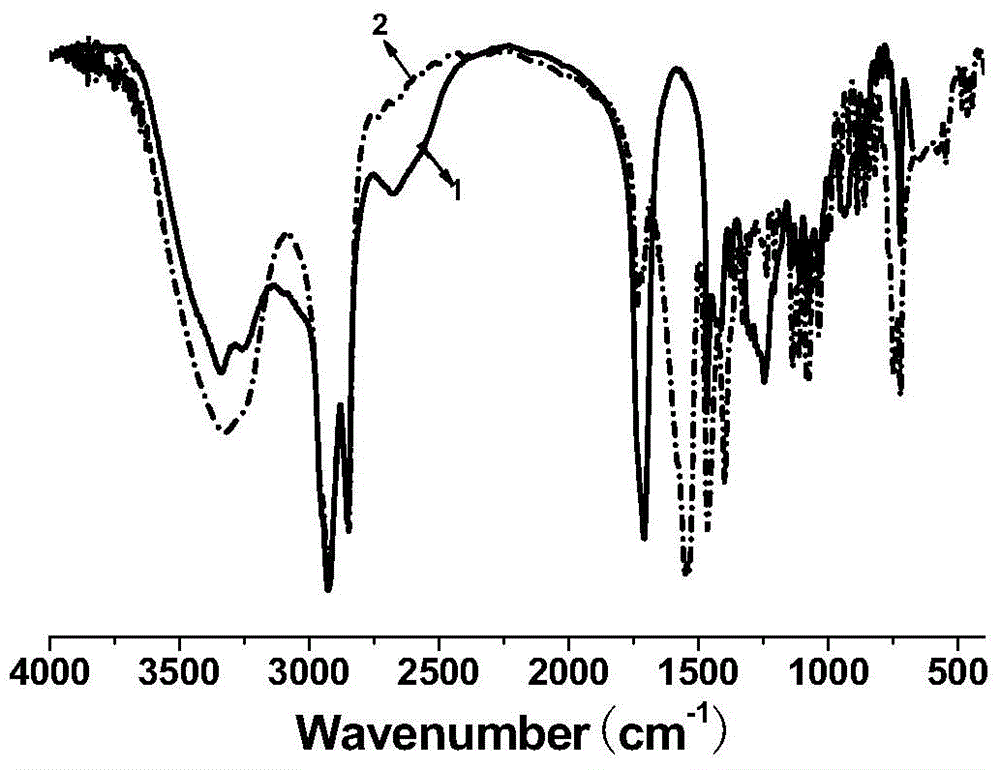
[0033] Add 11.0g of linoleic acid, 24.0g of industrial acetic acid (50% by mass fraction) and 4.0g of hydrochloric acid with 40% by mass fraction in a three-necked flask with stirring and a thermometer, and slowly add 30% by mass fraction to the system at room temperature. % hydrogen peroxide 24.0g, heated up to 55°C, and reacted for 6 hours under vigorous stirring. After the reaction was completed, it was filtered with suction, washed with hot water for 3 times, and then recrystallized with ethyl acetate to obtain a white powdery solid, namely tetrahydroxystearic acid, with a yield of 79.3%.
Embodiment 3
[0034] Embodiment 3: the preparation method of hexahydroxystearic acid
[0035] Add 10.8g of linolenic acid, 36.0g of industrial acetic acid (50% by mass fraction) and 4.0g of hydrochloric acid with 40% by mass fraction in a three-necked flask with stirring and a thermometer, and slowly dropwise add 30% by mass fraction to the system at room temperature 36.0 g of hydrogen peroxide was heated up to 60° C., and reacted for 6 hours under vigorous stirring. After the reaction was finished, it was suction filtered, washed with hot water for 3 times, and then recrystallized with ethyl acetate to obtain a white powdery solid which was hexahydroxystearic acid with a yield of 61.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com