A kind of synthetic method of polyhydroxystearate
A technology of polyhydroxystearate and polyhydroxystearic acid, which is applied in the direction of carboxylate preparation, carboxylate preparation, chemical instruments and methods, etc., can solve the problem of environmental pollution and 9,10-dihydroxystearic acid Post-processing complex, corrosive and other problems, to achieve the effect of expanding production capacity, shortening residence time, weakening corrosion and toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: the preparation method of dihydroxystearic acid
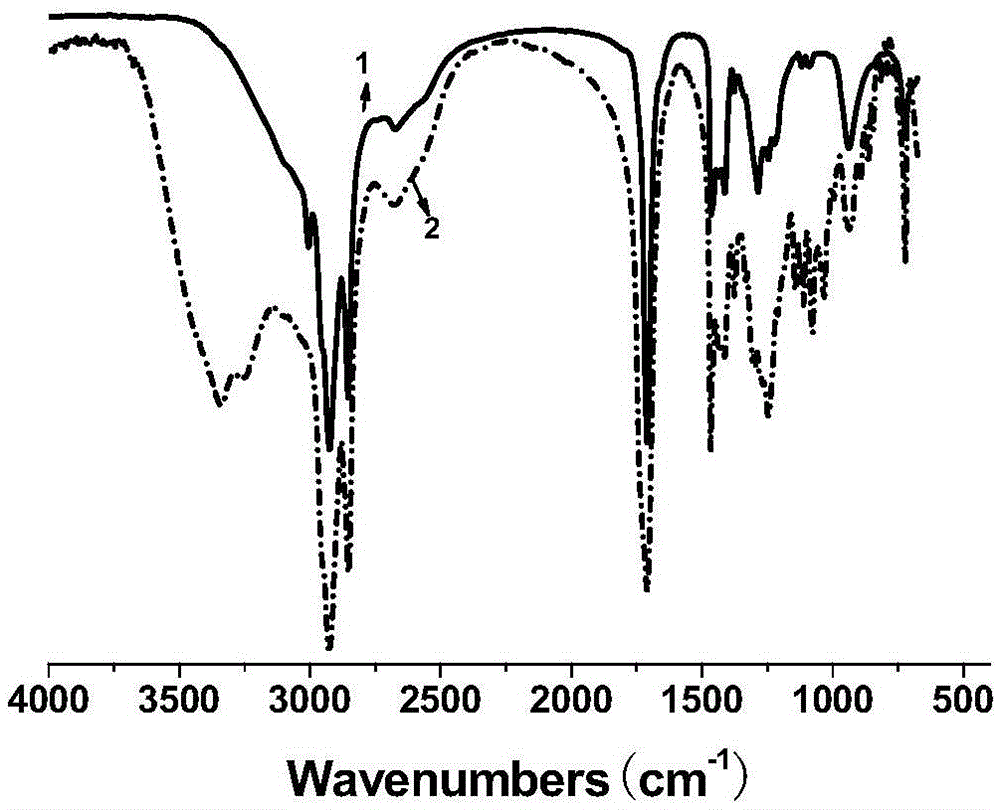
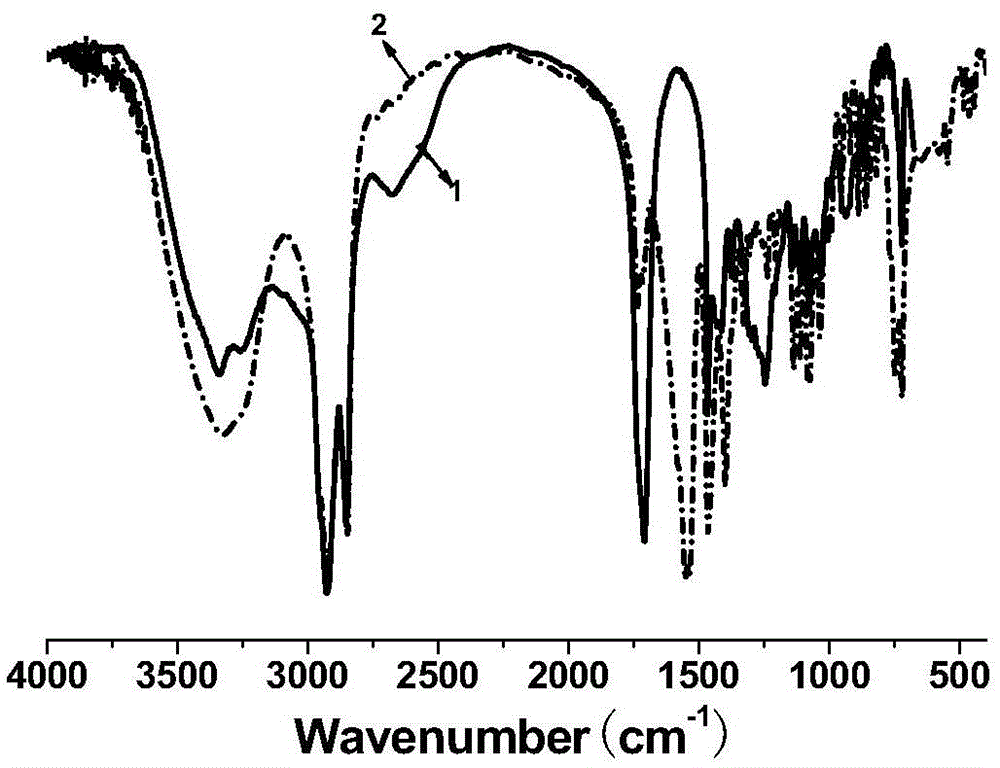
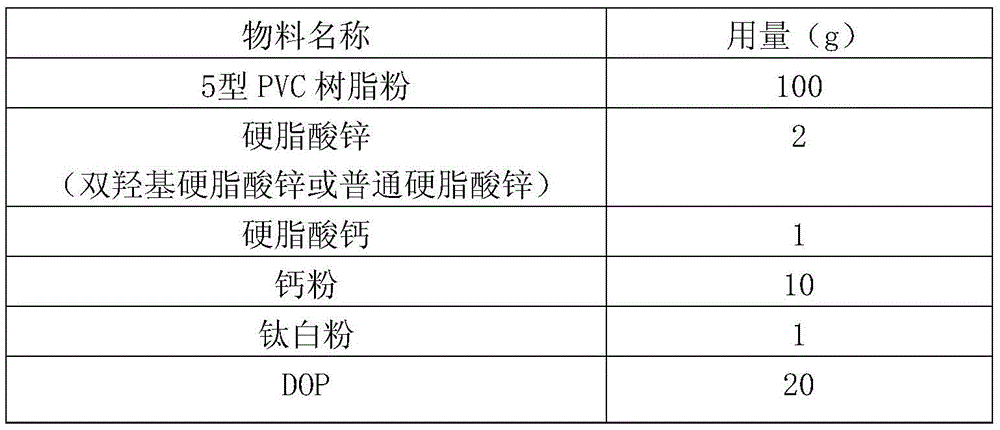
[0031] Add 11.3g of oleic acid, 16.0g of industrial acetic acid (50% by mass fraction) and 4.0g of hydrochloric acid with 40% by mass fraction in a three-necked flask with stirring and a thermometer, and slowly dropwise add 30% by mass fraction to the system at room temperature 12.0 g of hydrogen peroxide was heated up to 55° C., and reacted for 6 hours under vigorous stirring. After the reaction was completed, it was suction filtered, washed with hot water for 3 times, and then recrystallized with ethyl acetate to obtain a white powdery solid which was dihydroxystearic acid, with a melting point of 92-96° C. and a yield of 97%. Take the product for infrared spectroscopy detection, such as figure 1 As shown, the result is displayed at 3400cm -1 There is a relatively obvious peak at , which is attributed to the hydroxyl functional group in the molecular structure, so it can be judged that the hydroxyl func...
Embodiment 2
[0032]Embodiment 2: the preparation method of tetrahydroxystearic acid
[0033] Add 11.0g of linoleic acid, 24.0g of industrial acetic acid (50% by mass fraction) and 4.0g of hydrochloric acid with 40% by mass fraction in a three-necked flask with stirring and a thermometer, and slowly add 30% by mass fraction to the system at room temperature. % hydrogen peroxide 24.0g, heated up to 55°C, and reacted for 6 hours under vigorous stirring. After the reaction was completed, it was filtered with suction, washed with hot water for 3 times, and then recrystallized with ethyl acetate to obtain a white powdery solid, namely tetrahydroxystearic acid, with a yield of 79.3%.
Embodiment 3
[0034] Embodiment 3: the preparation method of hexahydroxystearic acid
[0035] Add 10.8g of linolenic acid, 36.0g of industrial acetic acid (50% by mass fraction) and 4.0g of hydrochloric acid with 40% by mass fraction in a three-necked flask with stirring and a thermometer, and slowly dropwise add 30% by mass fraction to the system at room temperature 36.0 g of hydrogen peroxide was heated up to 60° C., and reacted for 6 hours under vigorous stirring. After the reaction was finished, it was suction filtered, washed with hot water for 3 times, and then recrystallized with ethyl acetate to obtain a white powdery solid which was hexahydroxystearic acid with a yield of 61.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com