Synthesis method of dapagliflozin
A synthetic method and technology of synthetic route, applied in the direction of organic chemistry, etc., can solve the problem of unclear root cause, and achieve the effects of easy extraction and separation, high yield, and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
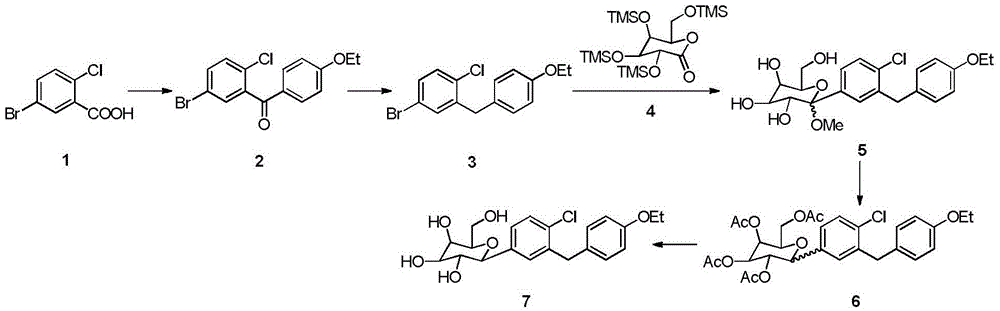
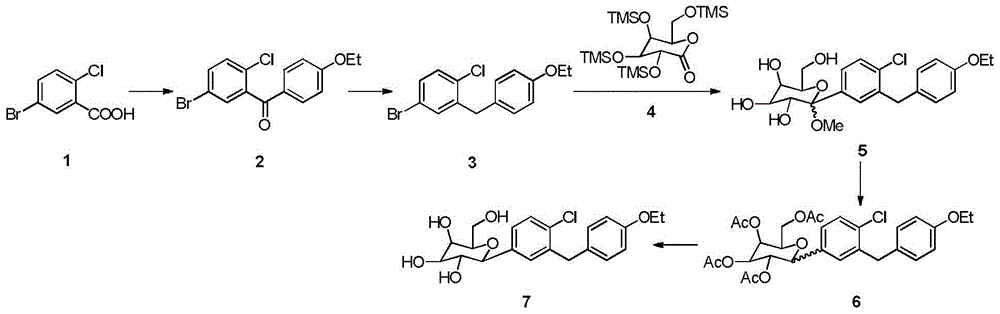
[0018] Embodiment 1: preparation compound 2
[0019] Add 50g of raw material 4, 0.5ml of anhydrous DMF and 250ml of DCM to the three-necked flask, add dropwise 30ml of oxalyl chloride (or 25ml of thionyl chloride), drop it for 5 hours, react for 3 hours, concentrate, add 100ml of DCM to the residue, and cool to -10~ 0°C, add 20g of phenethyl ether and 30g of anhydrous aluminum trichloride, after addition, react for 3h at 0°C, add the reaction solution into ice water, separate and extract, wash the organic phase with acid, alkali, water, dry, and concentrate to obtain 62.2 g compound 2, yield: 86.2%, purity 97.9%.
Embodiment 2
[0020] Embodiment 2: preparation compound 3
[0021] Add 50g of compound 2, 300ml of ethanol and 10g of sodium borohydride into the three-necked flask, react for 1 hour, then cool down to -10~0°C, add 50g of anhydrous aluminum trichloride, rise to 0~5°C for 5 hours, then reflux for 12 hours, Cooled, concentrated, added to ice water, extracted with EA, the organic phase was acid-washed, washed with water, dried, and concentrated to obtain 45.1 g of compound 3 with a yield of 94.1% and a purity of 95.2%.
Embodiment 3
[0022] Embodiment 3: preparation compound 5
[0023] Add 50g of compound 3 and 300ml THF to the three-necked flask, cool down to -78°C, add 120ml of 2mol / L butyllithium dropwise, stir for 1h, add the above reaction solution dropwise to 110g of compound 4 in toluene (260ml) solution, the reaction temperature React at -78°C for 3 hours, add 200ml of 1mol / L methanol solution of methanesulfonic acid dropwise, rise to room temperature for reaction, add sodium carbonate to adjust the pH value to 7-8, separate liquid extraction, wash the organic phase with water, dry and concentrate to obtain 68.1g Compound 5 has a crude yield of 101% and a purity of 92.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com