Method for extracting urease from jack beans
A technology of urease and sword bean, which is applied in the field of extraction and purification of urease through membrane separation, can solve the problems of prolonging the process implementation time, increasing the recovery cost, and increasing the extraction cost, so as to shorten the extraction and purification process, achieve high production safety, and occupy small area effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
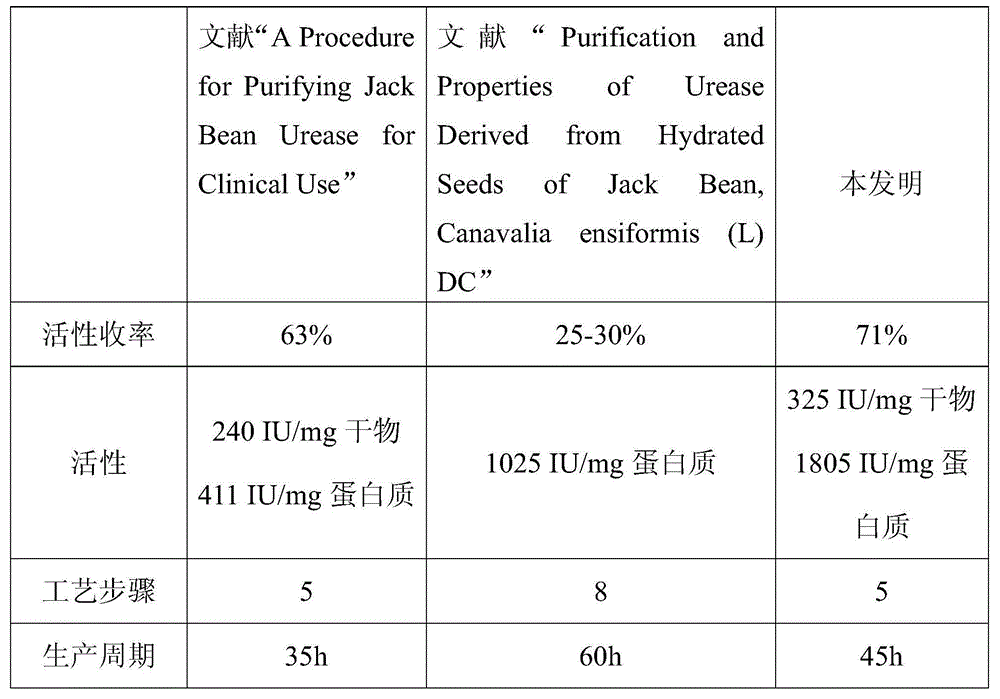
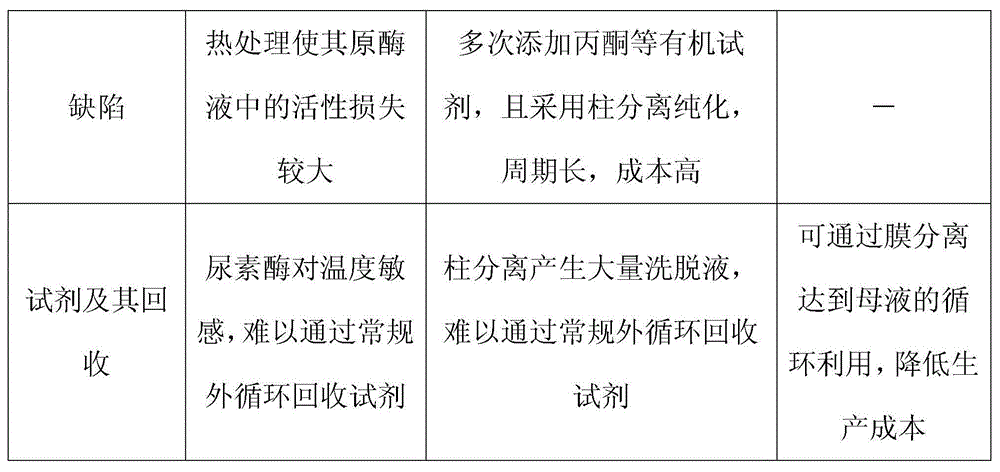
[0044] Take 50Kg of sword bean powder, add 150Kg of 0.05M Tris-Hcl buffer solution (including 30% acetone, 1mM EDTA and 1mM mercaptoethanol) at room temperature, stir and extract for 2 hours, centrifuge, collect the supernatant, and adjust the pH with citric acid to 6.2, refrigerated, collected the supernatant, then adjusted the pH to 5.2 with citric acid, refrigerated, and centrifuged to collect the precipitate. Add 0.1M pH 8.0 Tris-Hcl buffer solution to the above precipitate to neutralize it, centrifuge, and the precipitate is neutralized again in the same way, and a total of 39.6Kg of the neutralized solution is collected twice.
[0045]The neutralized solution was microfiltered through a 1200nm ceramic membrane, and 2Kg of distilled water was added each time for dialysis, and a total of 20% of the original solution (ie 7.9Kg) of the dialysate was added to obtain a total of 37.3Kg of the permeate and the dialysate, and a total of 8.7Kg of the concentrated solution. The abo...
Embodiment 2
[0047] Take 50Kg of sword bean powder, add 150Kg of 0.2M pH6.8 BTP buffer solution (including 30% acetone, 1mM EDTA and 1mM mercaptoethanol) to extract with stirring at room temperature for 0.5h, and centrifuge to collect the supernatant. Adjust the pH to 5.8 with hydrochloric acid, refrigerate, and centrifuge. The supernatant is then adjusted to pH 4.8 with hydrochloric acid, refrigerated, and centrifuged to collect the precipitate. Add 0.01M pH 7.2BTP buffer to the above precipitate to neutralize it, centrifuge, and neutralize the precipitate again in the same way, and collect a total of 42.9Kg of the neutralized solution twice.
[0048] Perform microfiltration through a 500nm ceramic membrane, add 2.1Kg of 0.01M BTP buffer each time for dialysis, add a total of 40% of the original solution of the dialysate (ie 17.2Kg), and obtain a total of 47.2Kg of the permeate and dialysate, and a total of 10.3Kg of the concentrate . Combine the above-mentioned permeate and dialysate an...
Embodiment 3
[0050] Take 50Kg of sword bean powder, add 500Kg of 0.1M pH7.0 phosphate buffer (including 30% acetone, 1mM EDTA and 1mM mercaptoethanol) and stir for extraction at room temperature for 1h, centrifuge and collect the supernatant. Adjust the pH to 6.0 with acetic acid, refrigerate, and centrifuge. The supernatant is then adjusted to pH 5.0 with acetic acid, refrigerated, and centrifuged to collect the precipitate. Add 0.02M pH7.5 phosphate buffer to the above precipitate to neutralize it, centrifuge, and neutralize the precipitate again in the same way, and collect a total of 94.7Kg of the neutralized solution twice.
[0051] The neutralized solution was microfiltered through an 800nm ceramic membrane, and 4.7Kg of distilled water was added each time for dialysis. A total of 30% of the stock solution (ie 28.4Kg) was added to obtain a total of 101.3Kg of the permeate and the dialysate, and a total of 16.3Kg of the concentrated solution. The above-mentioned permeate and dialyza...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com