Hyperthermophilic glycosidase mutant and application thereof in preparation of ginsenoside CK
A thermophilic glycosidase and mutant technology, applied in the field of biotechnology engineering, can solve the problems of difficulty in artificial cultivation of thermophilic bacteria, low glycosidase content, unfavorable production of thermostable glycosidase, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
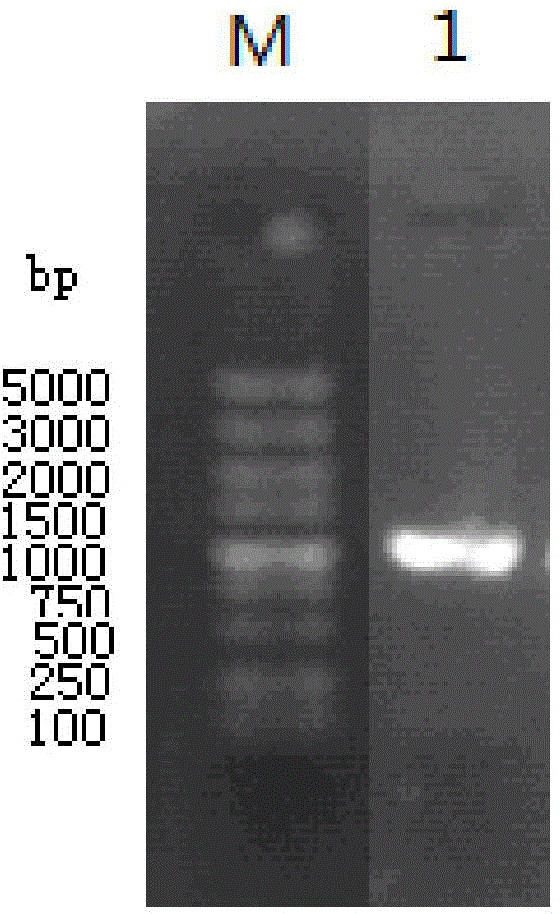
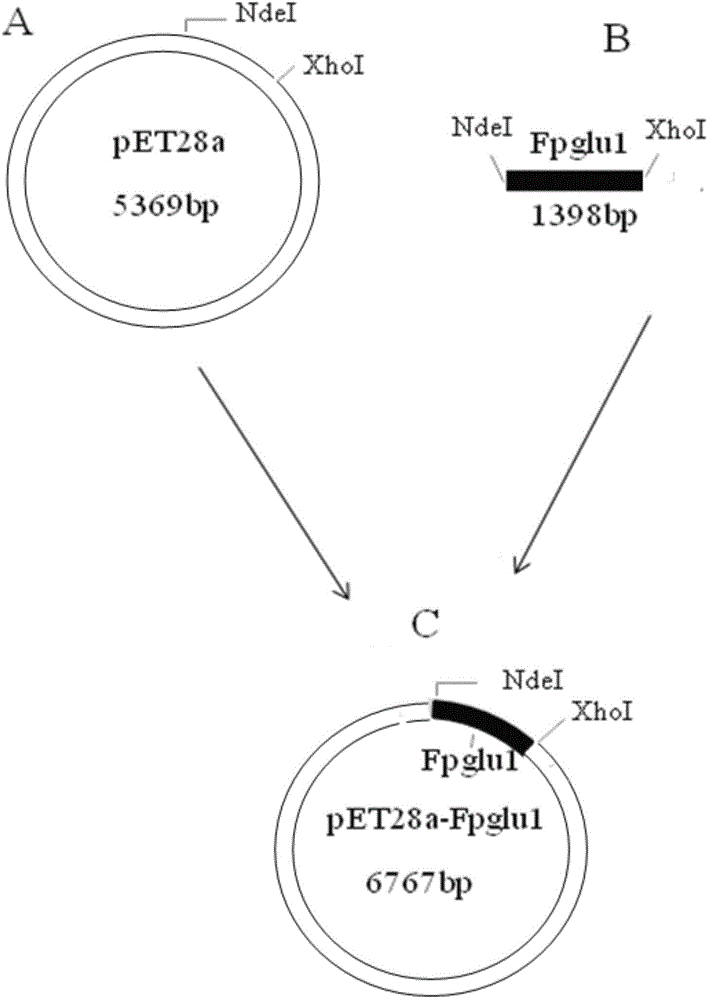
[0030]The construction of embodiment 1 thermophilic bacterium glycosidase engineering bacteria and its enzyme expression
[0031] (1) Cultivation of thermophilic bacteria Fervidobacterium pennivorans DSM 9078 and extraction of chromosomal DNA
[0032] Thermophilic bacteria Fervidobacterium pennivorans DSM 9078 was purchased from DSMZ (Germany). The composition is as follows: NH 4 C1, 0.5g; MgSO 4 ×7H 2 O, 0.16g; KH 2 PO 4 , 1.6g; Na 2 HPO 4 ×H 2 O, 1.0g; CaCl 2 ×2H 2 O, 0.06g; trace mineral solution, 10ml; Vitamin solution, 10ml; yeast extract, 2g; trypticase, 2g; resazurin, 0.5mg; glucose, 3g; Cysteine-HCl×H 2 O, 0.3g; Na 2 S×9H 2 O, 0.3 g. The composition of each liter of trace mineral solution is as follows: nitrilotriacetic acid, 1.5g; MgSO 4 ×7H 2 O, 3g; MnSO 4 ·H 2 O, 0.5g; NaCl, 1.0g; FeSO 4 ·7H 2 O, 0.1g; CoSO4 7H 2 O, 0.18g; CaCl 2 2H 2 O, 0.1g; ZnSO 4 ×7H 2 O, 0.18g; CuSO 4 ×5H 2 O, 0.01g; KAl(SO 4 ) 2 ×12H 2 O, 0.02g; H 3 BO 3 , 0.01g;...
Embodiment 2
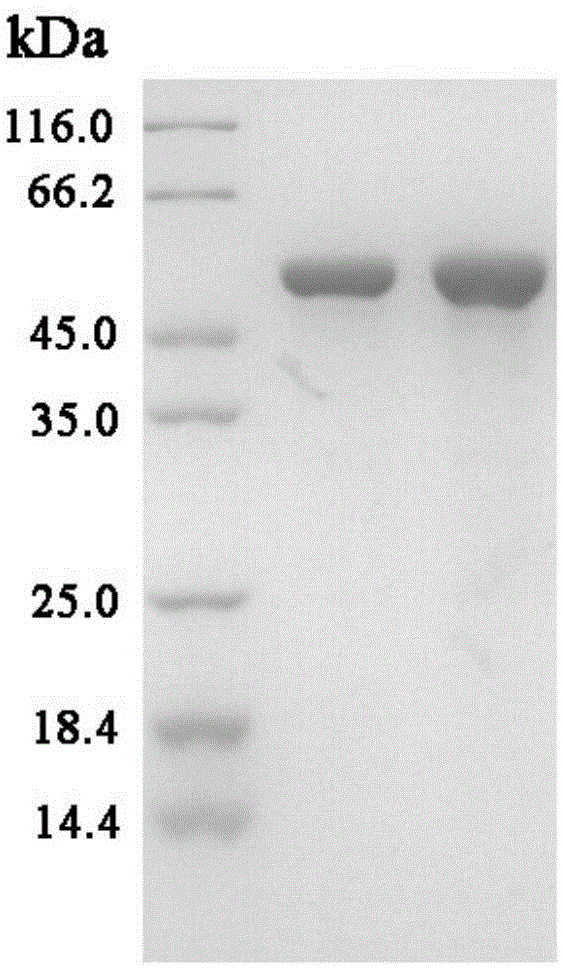
[0054] The characteristics of embodiment 2 hyperthermophilic recombinant glycosidase mutants
[0055] (1) Substrate specificity of wild-type and mutant glycosidases
[0056] Various p-nitrophenol glycoside substrates were weighed, and the enzyme activity was measured referring to the glycosidase measurement conditions described in (1).
[0057] It can be seen from Table 1 that thermophilic glucosidase and its mutants showed different hydrolysis activities to a series of p-nitrophenol glycoside substrates, among which the hydrolysis activity of p-nitrophenyl-β-D-glucoside was the highest, and the mutants hydrolyzed The activity of p-nitrophenyl-β-D-glucoside is about 2.5 times that of the wild type.
[0058] Table 1 Substrate selectivity of wild-type hyperthermophilic glycosidase and its mutants
[0059]
[0060] (2) Comparison of conversion rates of wild-type and hyperthermophilic glycosidase mutant glycosidases for substrate-specific catalysis of ginsenoside conversion ...
Embodiment 3
[0071] Example 3 Transformation of rare ginsenoside CK by thermophilic glycosidase Fpglu1a
[0072] Add 1mL of 1mg / ml solution of ginsenosides Rb1, Rb2 and Rc, and then add an equal volume of the purified enzyme solution obtained above, and react at 70°C for 12 hours. After the reaction, add an equal volume of water-saturated n-butyl Alcohol terminated the reaction, vortexed and mixed, centrifuged at 8000rpm for 2min, and after standing for 5min, took the upper n-butanol layer solution, evaporated to dryness in a water bath at 60°C, collected solid samples, and used chromatographic grade methanol to dilute to 1ml to obtain the total saponins of ginseng root. The converted samples were filtered with a 0.22 μm organic membrane and used for HPLC detection. The detection condition of high performance liquid chromatography is, C 18 Reverse-phase chromatographic column (5 cm×3.0 mm, 2.7 μm; Supelco. USA). Mobile phase is water (A) and acetonitrile (B), gradient elution conditions:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com