Pharmaceutical composition for treating bovine lithangiuria and preparation method of pharmaceutical composition
A composition and technology of road stones, applied in the field of traditional Chinese veterinary medicine, can solve the problems of large toxic and side effects, promote the formation of uroliths, and cause great harm, and achieve the effect of enhancing immunity, improving resistance, and strong pertinence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
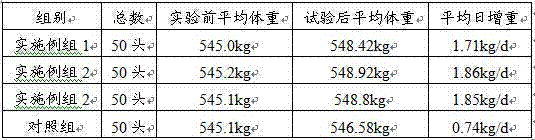
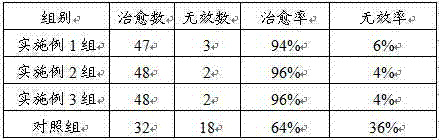
Image
Examples
Embodiment 1
[0067] Example 1 powder
[0068] 35 parts of Qumai, 40 parts of Lonicerae vine, 10 parts of talc, 10 parts of Abutilon hempseed, 20 parts of licorice, 14 parts of golden cherry root, 25 parts of Centella asiatica, 3 parts of Clematis, 10 parts of black herb, 30 parts of Spatholobus, network 14 parts of stone vine, 20 parts of Qingfeng vine, 10 parts of sea wind vine, 8 parts of night cross vine, 25 parts of loofah, 25 parts of sea gold sand, 20 parts of turmeric, 20 parts of radish seed, 3 parts of Chuan Toosendan, 14 parts of corydalis 40 parts of white peony, 25 parts of angelica, 10 parts of ebony, 13 parts of earth yuan, 7 parts of blood, 14 parts of catechu, 20 parts of rhizoma rhizome, 25 parts of vine, 30 parts of stone swallow, and 20 parts of golden kidney.
[0069] Its preparation process is:
[0070] The first step is to mix the raw medicinal materials in proportion, add ethanol with an alcohol concentration of 95% that is 4 times the mass of the mixture, heat and ...
Embodiment 2
[0073] Example 2 Injection
[0074] 35 parts of Qumai, 40 parts of Lonicerae vine, 10 parts of talc, 10 parts of Abutilon hempseed, 20 parts of licorice, 14 parts of golden cherry root, 25 parts of Centella asiatica, 3 parts of Clematis, 10 parts of black herb, 30 parts of Spatholobus, network 14 parts of stone vine, 20 parts of Qingfeng vine, 10 parts of sea wind vine, 8 parts of night cross vine, 25 parts of loofah, 25 parts of sea gold sand, 20 parts of turmeric, 20 parts of radish seed, 3 parts of Chuan Toosendan, 14 parts of corydalis 40 parts of white peony root, 25 parts of angelica, 10 parts of ebony plum, 13 parts of earth yuan, 7 parts of blood dried fruit, 14 parts of catechu, 20 parts of rhizoma rhizome, 25 parts of shield wing rattan, 30 parts of stone swallow, 20 parts of golden kidney, Lian Qiancao 14 parts, Plantago Seed 10 parts, Guan Mu Tong 8 parts, Xu Changqing 20 parts, Shi Wei 10 parts, Creeping Dragon 25 parts, White Green Leaf 10 parts.
[0075] Its pr...
Embodiment 3
[0079] Embodiment 3 Decoction
[0080] 15 parts of Qumai, 40 parts of Lonicerae vine, 10 parts of talc, 10 parts of Abutilus hempseed, 20 parts of licorice, 10 parts of golden cherry root, 25 parts of Centella asiatica, 3 parts of Clematis, 10 parts of black herb, 30 parts of Spatholobus, network 14 parts of stone vine, 10 parts of Qingfeng vine, 13 parts of sea wind vine, 12 parts of night cross vine, 25 parts of loofah, 20 parts of sea gold sand, 10 parts of turmeric, 15 parts of radish seed, 3 parts of chuansendan, 10 parts of corydalis 40 parts of white peony root, 25 parts of angelica, 10 parts of ebony plum, 13 parts of earth yuan, 7 parts of blood dried fruit, 10 parts of catechu, 30 parts of rhizoma rhizome, 25 parts of scutellaria, 20 parts of stone swallow, 20 parts of golden kidney, Lian Qiancao 14 parts, Plantago Seed 20 parts, Guan Mu Tong 8 parts, Xu Changqing 10 parts, Shi Wei 10 parts, Crouching Dragon 30 parts, and White Green Leaf 20 parts.
[0081] Its prep...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com