Anti-HPV (human papillomavirus) gel dressing and preparation method thereof
An HPV virus and gel technology, which is applied in the field of medicine, can solve the problems of not being easy to penetrate deep into the cavity, affecting the effect of drugs, and poor stability, and achieving the treatment of HPV virus infection, good clinical application prospects, and direct drug effects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
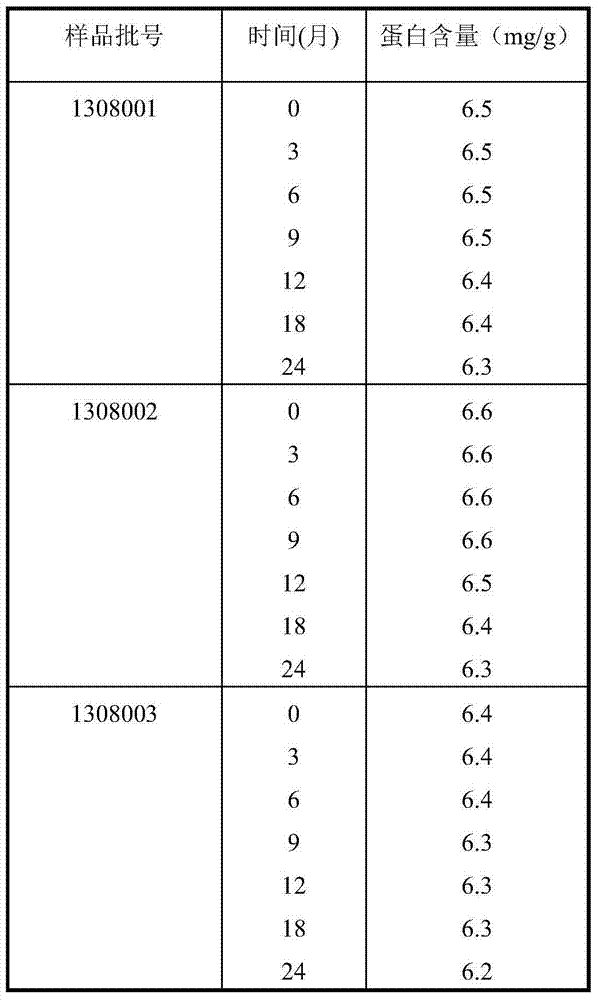
[0043] 1. Preparation of tea acidic protein AP
[0044] Take fresh tea leaves from Wuzhi Mountain in Hainan, grind and homogenize them with a material-water ratio of 1:5 (w / v), adjust the pH to 7-9, extract at 40°C for 3 hours, filter and centrifuge, take the supernatant, adjust the pH to 2-5, centrifuge, and take the precipitate , and the crude product of tea acidic protein AP was obtained after freeze-drying. After the crude tea acidic protein AP is reconstituted, it passes through a CM Sepharose Fast Flow chromatography column to remove the basic protein, collects the flow-through solution containing the acidic protein, and vacuum freeze-dries it to make the tea acidic protein AP.
[0045] 2. Preparation of anti-HPV virus freeze-dried powder:
[0046]Add 0.01 part of tea acid protein AP, 10 parts of maltose isopolysaccharide, 89.94 parts of polysaccharide, and 0.05 part of chlorhexidine, add water to dissolve, stir evenly, and dry in vacuum at low temperature.
[0047] 3....
Embodiment 2
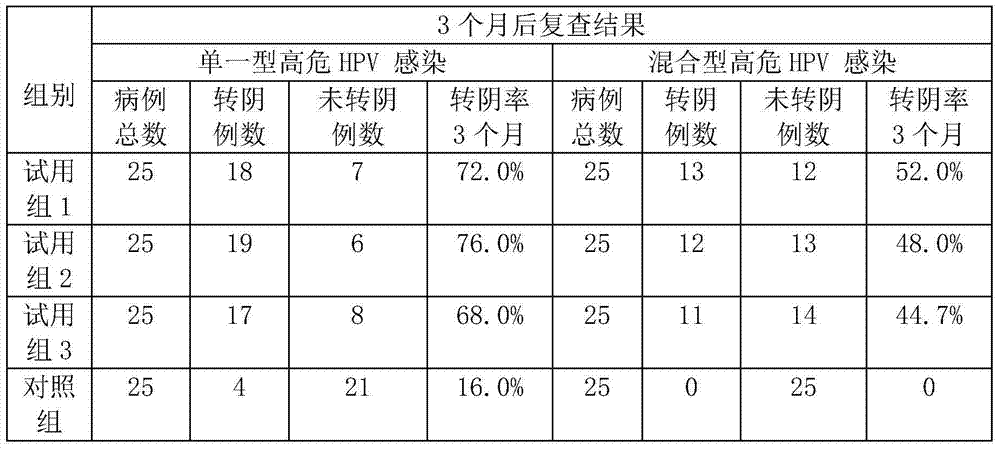
[0056] 1. Preparation of tea acidic protein AP
[0057] Take fresh tea leaves from Wuzhi Mountain in Hainan, grind and homogenize them with a material-water ratio of 1:8 (w / v), adjust the pH to 7-9, extract at 25°C for 3 hours, filter and centrifuge, take the supernatant, adjust the pH to 2-5, centrifuge, and take the precipitate , and the crude product of tea acidic protein AP was obtained after freeze-drying. After the crude tea acidic protein AP is reconstituted, it passes through a CM Sepharose Fast Flow chromatography column to remove the basic protein, collects the flow-through solution containing the acidic protein, and vacuum freeze-dries it to make the tea acidic protein AP.
[0058] 2. Preparation of anti-HPV virus freeze-dried powder:
[0059] Add 1 part of tea acid protein AP, 15 parts of maltose, 83.99 parts of polysaccharide, and 0.01 part of chlorhexidine, dissolve in water, stir evenly, and dry in vacuum at low temperature.
[0060] 3. Preparation of solution...
Embodiment 3
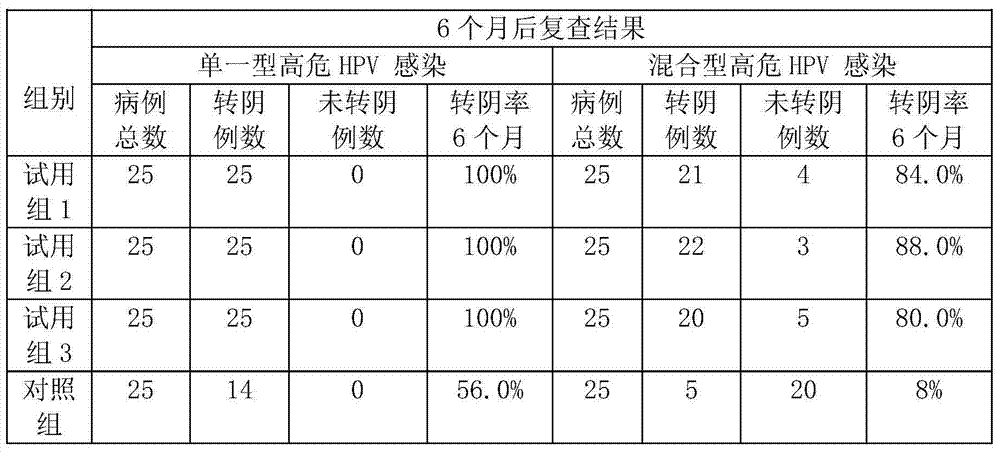
[0069] 1. Preparation of tea acidic protein AP
[0070] Take fresh tea leaves from Wuzhi Mountain in Hainan, grind and homogenize them with a material-water ratio of 1:10 (w / v), adjust the pH to 7-9, extract at 10°C for 3 hours, filter and centrifuge, take the supernatant, adjust the pH to 2-5, centrifuge, and take the precipitate , and the crude product of tea acidic protein AP was obtained after freeze-drying. After the crude tea acidic protein AP is reconstituted, it passes through a CM Sepharose Fast Flow chromatography column to remove the basic protein, collects the flow-through solution containing the acidic protein, and vacuum freeze-dries it to make the tea acidic protein AP.
[0071] 2. Preparation of anti-HPV virus freeze-dried powder:
[0072] Add 5 parts of tea acid protein AP, 25 parts of maltose, 69.98 parts of polysaccharide, and 0.02 part of chlorhexidine, add water to dissolve, stir evenly, and dry in vacuum at low temperature.
[0073] 3. Preparation of so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com