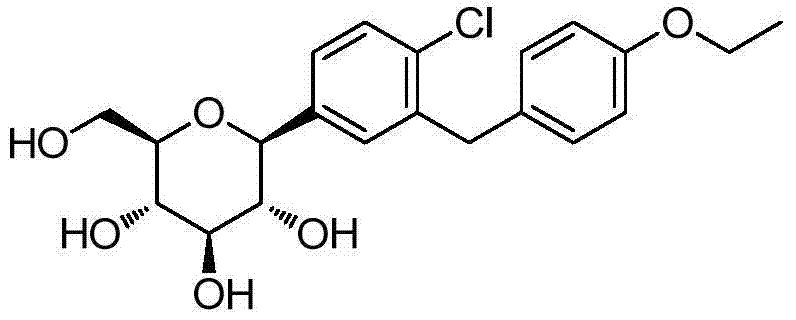
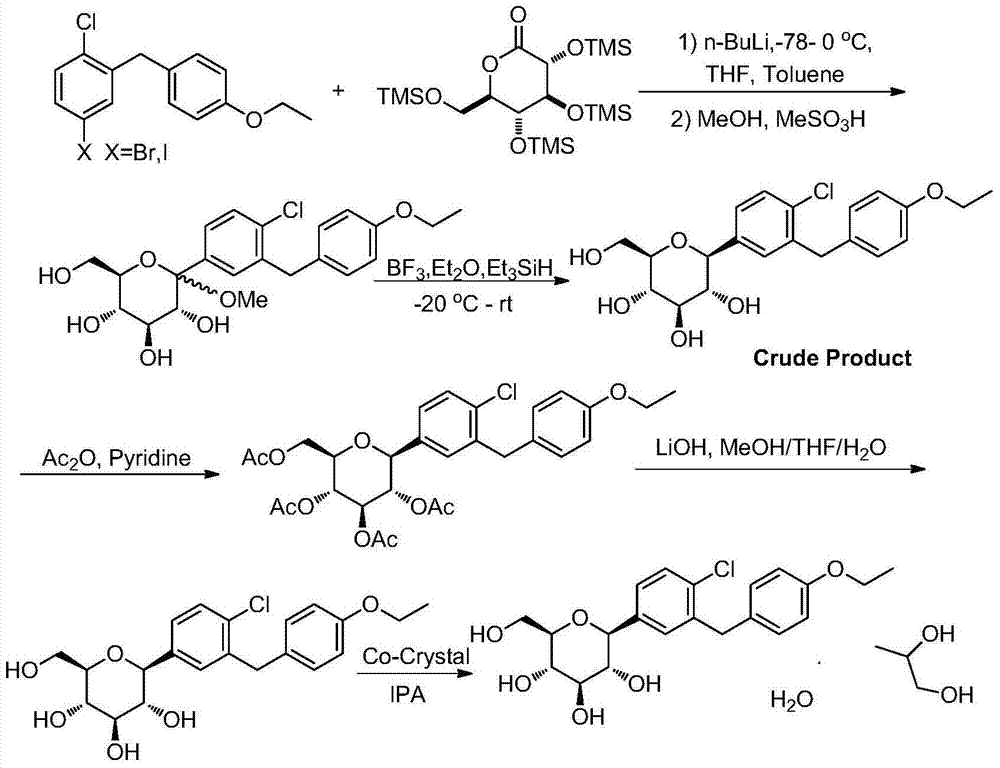
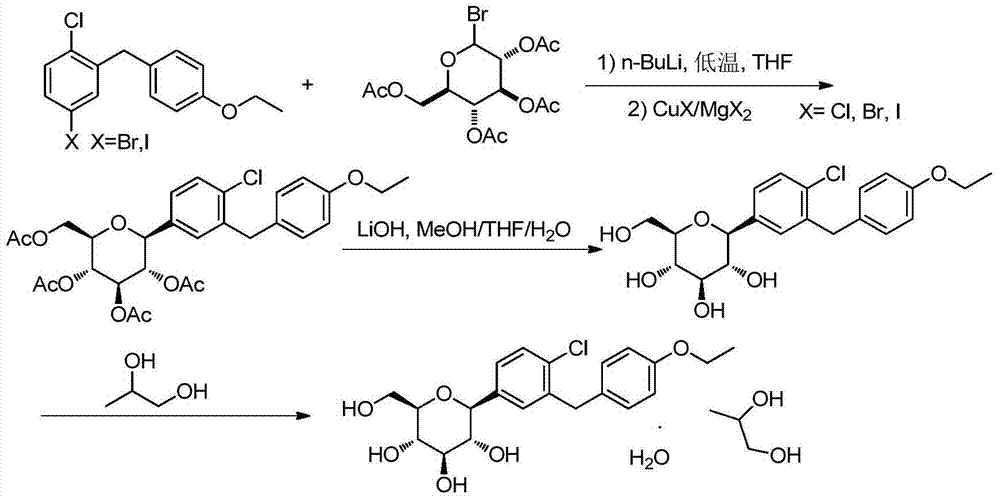
Synthesis method of dapagliflozin
A production method and compound technology, applied in the field of dapagliflozin synthesis, can solve the problems of high cost, strong hygroscopicity of zinc bromide, unfavorable amplification and operation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: the preparation of compound 1
[0034]
[0035] Under the condition of argon protection, add 300mL of dried tetrahydrofuran (THF) and 65.5g (200mmol) of 5-bromo-2-chloro-4-ethoxydiphenylmethane into a 1L three-necked flask. Bath to control the temperature of the reaction system at -78°C, slowly add n-butyl lithium (n-BuLi) 80mL (2.5mol / L, 200mmol, 1eq) dropwise, control the temperature of the reaction system below -78°C , after the dropwise addition, continue to keep at -78°C for 1 hour;
[0036] Then slowly add 19.1g (100mmol) of cuprous iodide to the reaction system, control the temperature of the reaction system at -40~-30°C for 1 hour, then slowly add 2,3,4, 6-tetra-O-acetyl-α-D-glucopyranose THF solution (41.5g, 100mmol, THF 100mL, 0.5eq), control the reaction temperature at -40~-30°C during the addition process and keep the temperature React for 1 hour, and slowly return the temperature of the reaction system to room temperature and react for 3 ...
Embodiment 2
[0040] Embodiment 2: the preparation of compound 1
[0041]
[0042] Under the condition of argon protection, 350mL of dried THF and 163.1g (500mmol) of 5-bromo-2-chloro-4-ethoxydiphenylmethane were added to a 1L three-necked flask, and the reaction was decomposed with acetone / dry ice bath. The temperature of the system is controlled at -60°C, and 240mL (2.5mol / L, 600mmol, 1.2eq) of n-butyllithium is slowly added dropwise. During the dropping process, the temperature of the reaction system is controlled below -60°C. React at -60°C for 1 hour;
[0043] Then slowly add 24.2g (240mmol) of cuprous iodide to the reaction system, control the temperature of the reaction system at -30~-20°C for 1 hour, then slowly add 2,3,4, 6-tetra-O-acetyl-α-D-glucopyranose in THF solution (98.8g, 240mmol, THF 150mL, 1.0eq), control the reaction temperature at -30~-20°C during the addition process and keep the temperature React for 1 hour, slowly return the temperature of the reaction system to...
Embodiment 3
[0047] Embodiment 3: the preparation of compound 1
[0048]
[0049] Under the condition of argon protection, 800mL of dried THF and 99.2g (266mmol) of 5-iodo-2-chloro-4-ethoxydiphenylmethane were added to a 2L three-necked flask, and the reaction was carried out in an acetone / dry ice bath. The temperature of the system is controlled at -50°C, and 160mL (2.5mol / L, 400mmol, 1.5eq) of n-butyllithium is slowly added dropwise. During the dropping process, the temperature of the reaction system is controlled at -50°C. React at 50°C for 1 hour;
[0050] Then slowly add 49.1g (100mmol) of magnesium bromide to the reaction system, control the temperature of the reaction system at -20~-10°C for 1 hour, then slowly add 2,3,4,6 -Tetra-O-acetyl-α-D-glucopyranose THF solution (98.5g, 240mmol, THF 200mL, 0.9eq), control the reaction temperature at -10~0°C during the addition process and keep the temperature for reaction 1 hour, slowly return the temperature of the reaction system to ro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com