Anthracycline bitriazole and copper fluoroborate complex with 4-pyridinylphenylboronic acid catalyzing effect and preparation method of anthracycline bitriazole and copper fluoroborate complex
A technology of anthracycline bistriazole copper and copper complexes, which is applied in the field of inorganic synthesis, can solve the problems of expensive palladium catalysts, and achieve the effects of being suitable for large-scale production, high reaction yield, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of 1-[9-(1H-1,2,4-triazol-1-yl)anthracen-10-yl]-1H-1,2,4-triazole (tatrz) ligand
[0029] Using the "one-pot method", the organic compound was prepared by heating 9,10-dibromoanthracene, triazole, potassium carbonate and copper oxide in DMF polar solvent; wherein 9,10-dibromoanthracene: three Azole: potassium carbonate: the molar ratio of copper oxide is 2:10:30:1;
[0030]
[0031] The reaction temperature is 1500°C, and the reaction time is 18 hours.
Embodiment 2
[0033] Cu(BF 4 ) 2 and 1-[9-(1H-1,2,4-triazol-1-yl)anthracen-10-yl]-1H-1,2,4-triazole (tatrz) in a molar ratio of 1: 1;
[0034] tatrz (0.0624 g, 0.2 mmol) and Cu(BF 4 ) 2 (0.0691 g, 0.2 mmol) in H 2 O (6 mL) and CH3 CN (4 mL) was stirred in a mixed solvent at room temperature for half an hour and then filtered, and the filtrate was volatilized at room temperature to form yellow rod-shaped crystals analyzed by X-ray single crystal diffraction. Yield: 35% (calculated based on tatrz). Elemental analysis (C 36 h 28 B 2 CuF 8 N 12 o 2 ) Theoretical value (%): C, 48.16; H, 3.14; N, 18.72. Found: C, 48.15; H, 3.16; N, 18.69.
Embodiment 3
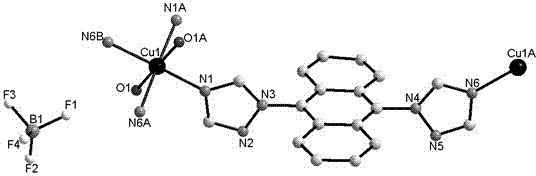
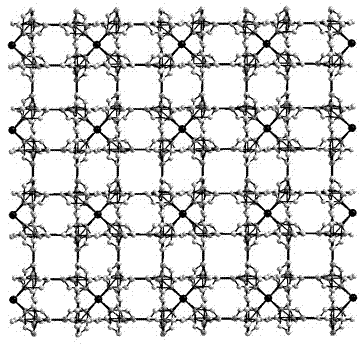
[0036] The crystal structure was determined using an APEX II CCD single crystal diffractometer, using graphite monochromatized Mokα rays (λ = 0.71073 ?) as the incident radiation. ω -2 θ Diffraction points are collected by scanning, and the unit cell parameters are obtained by least square method correction. The crystal structure is solved by software from the difference Fourier electron density map, and corrected by Lorentz and polarization effects. All H atoms were synthesized by difference Fourier transform and determined by ideal position calculation. The detailed crystal determination data are shown in Table 1. Structural primitives see figure 1 , the three-dimensional structure of the complex see figure 2 .
[0037] Table 1. Crystallographic data of complex 1
[0038]
[0039] Example 3
[0040] Cu(BF 4 ) 2 and 1-[9-(1H-1,2,4-triazol-1-yl)anthracen-10-yl]-1H-1,2,4-triazole (tatrz) in a molar ratio of 1: 1;
[0041] We also tried other ratios such as Cu(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com