Method for producing 4-hydroxycinnamaldehyde by catalyzing recombinant strain and whole cells thereof
A technology of recombinant strains and hydroxycinnamic acid, applied in the direction of microorganism-based methods, biochemical equipment and methods, microorganisms, etc., can solve the problems of catalyzed production of 4-hydroxycinnamaldehyde without recombinant bifunctional enzyme strains, and achieve reduction and complexity Sexuality, simplification of production process, low nutrition effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
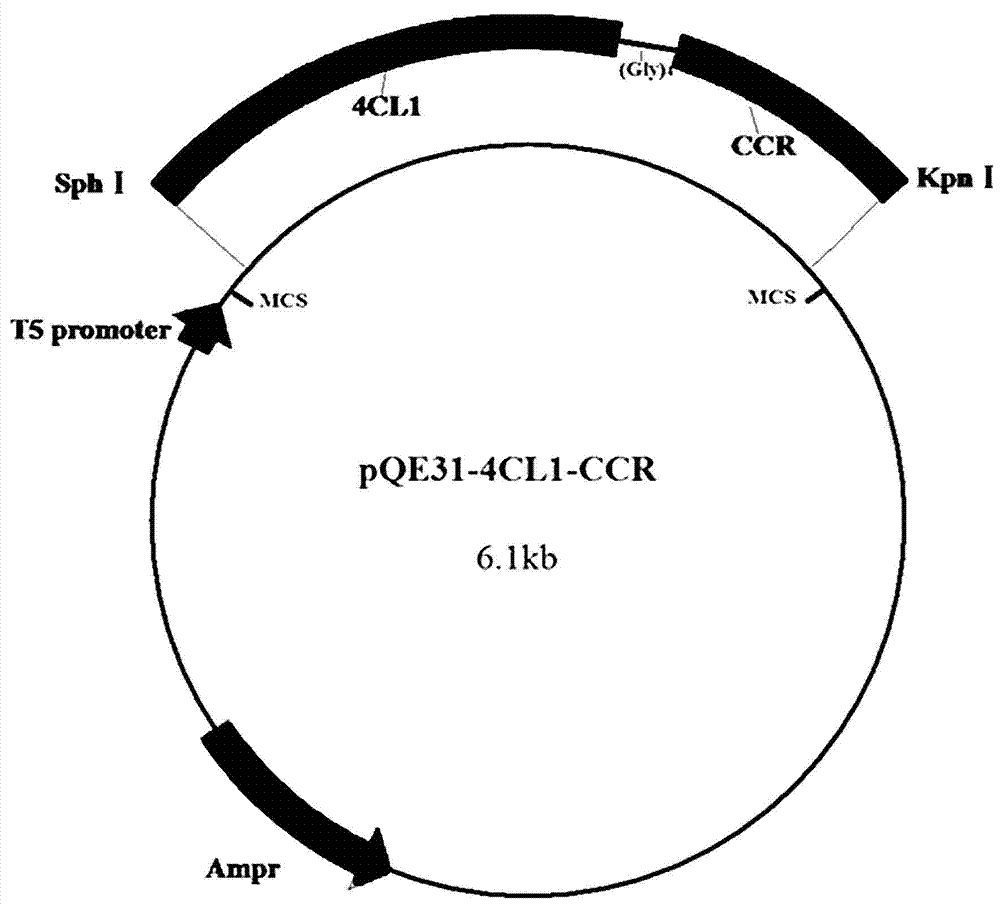
[0038] (1) Pick the recombinant strain E. coli M15 / pQE31-4CL1-CCR single colony was inoculated in 5mL LB liquid medium, and cultured overnight at 37°C and 200rpm with shaking; the obtained bacterial liquid was transferred to 50mL LB liquid culture at a volume ratio of 2:100 Shake at 37°C and 200rpm until the control OD600 is 0.6; add inducer isopropyl-β-D-thiogalactopyranoside IPTG to the culture to a final concentration of 0.4mmol / L, at 28°C, 200rpm induction culture for 8h; the formula of the LB liquid medium is: in g / L, tryptone 10, yeast extract 5, NaCl10, pH7.5, ampicillin 0.1, kanamycin 0.025; after stirring and dissolving evenly Sterilized and cooled for later use;
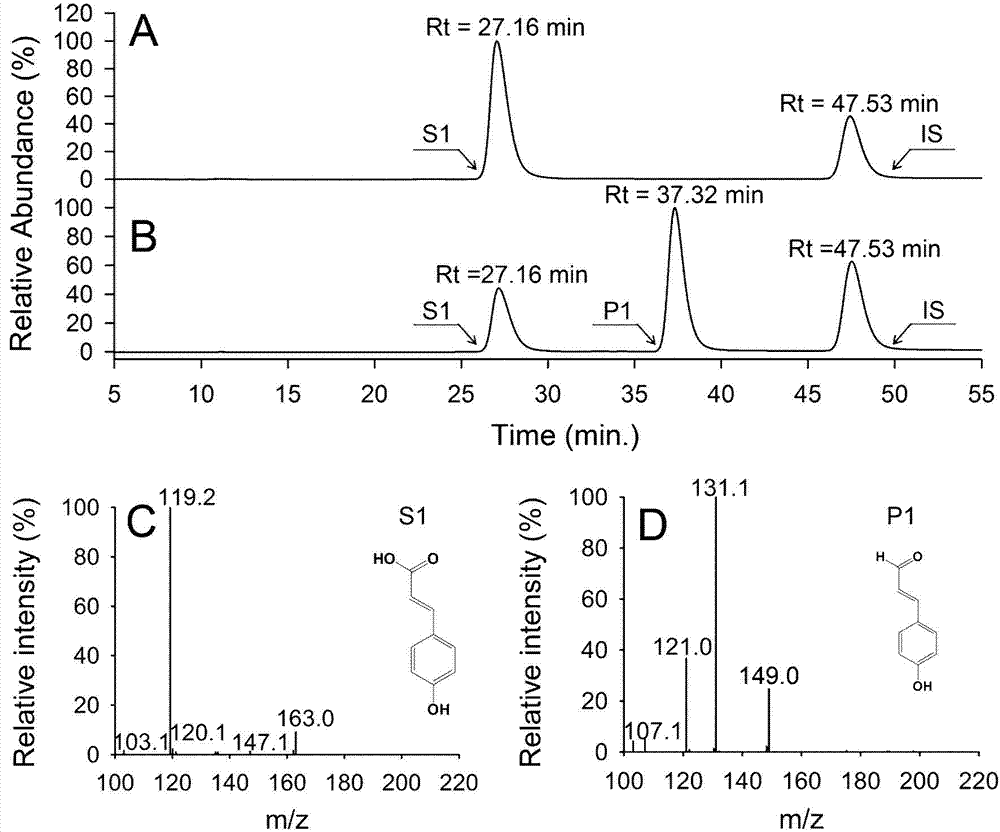
[0039] (2) Put the substrate coumaric acid at a concentration of 1mM directly into 50mL of the above liquid culture, and shake and react at 37°C and 200rpm in the dark for 4h;
[0040] (3) After the reaction is completed, centrifuge the cells at 4°C and 50×100 rpm to collect the reaction solution; the r...
Embodiment 2
[0042] (1) Pick the recombinant strain E. coli M15 / pQE31-4CL1-CCR single colony was inoculated in 5mL LB liquid medium, and cultured overnight at 37°C and 200rpm with shaking; the obtained bacterial liquid was transferred to 50mL LB liquid culture at a volume ratio of 2:100 Shake at 37°C and 200rpm until the control OD600 is 0.6; add inducer isopropyl-β-D-thiogalactopyranoside IPTG to the culture to a final concentration of 0.4mmol / L, at 28°C, 200rpm induction culture for 8h; the formula of the LB liquid medium is: in g / L, tryptone 10, yeast extract 5, NaCl10, pH7.5, ampicillin 0.1, kanamycin 0.025; after stirring and dissolving evenly Sterilized and cooled for later use;
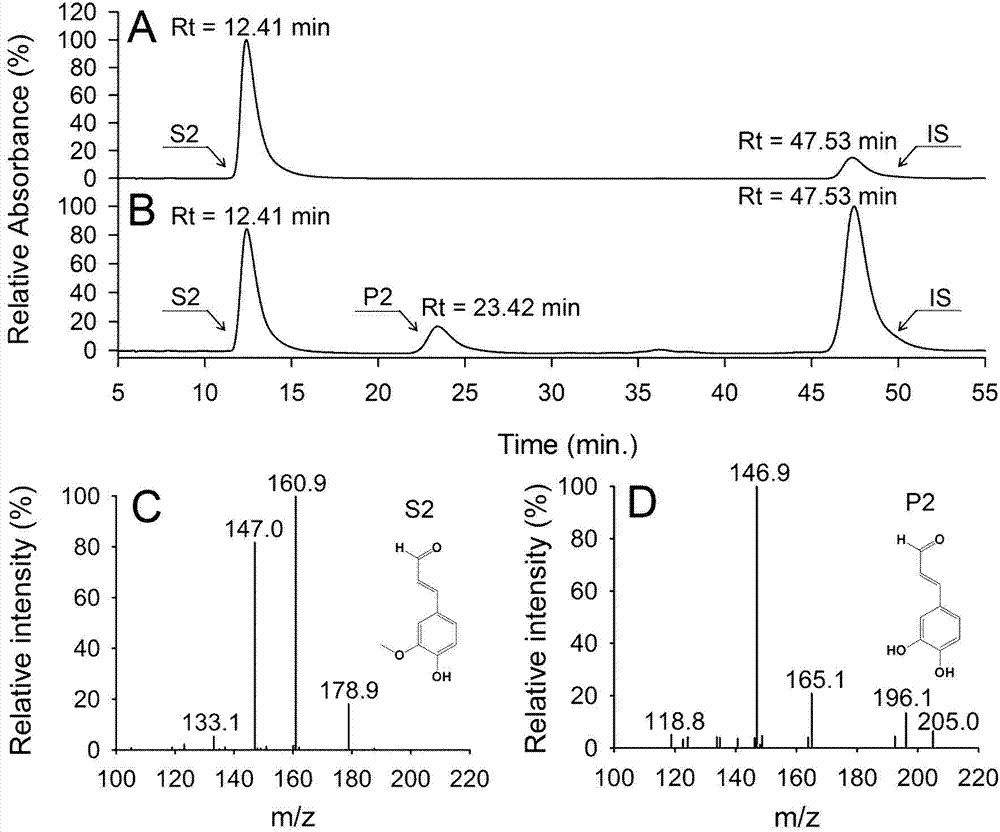
[0043] (2) Put the substrate caffeic acid at a concentration of 1mM directly into 50mL of the above liquid culture, and shake and react at 37°C and 200rpm in the dark for 8h;
[0044] (3) After the reaction is completed, centrifuge the cells at 4°C and 50×100 rpm to collect the reaction solution; the re...
Embodiment 3
[0046] (1) Pick the recombinant strain E. coli M15 / pQE31-4CL1-CCR single colony was inoculated in 5mL LB liquid medium, and cultured overnight at 37°C and 200rpm with shaking; the obtained bacterial liquid was transferred to 50mL LB liquid culture at a volume ratio of 2:100 Shake at 37°C and 200rpm until the control OD600 is 0.6; add inducer isopropyl-β-D-thiogalactopyranoside IPTG to the culture to a final concentration of 0.4mmol / L, at 28°C, 200rpm induction culture for 8h; the formula of the LB liquid medium is: in g / L, tryptone 10, yeast extract 5, NaCl10, pH7.5, ampicillin 0.1, kanamycin 0.025; after stirring and dissolving evenly Sterilized and cooled for later use;
[0047] (2) Put the substrate ferulic acid at a concentration of 1mM directly into 50mL of the above-mentioned liquid culture, and shake and react at 37°C and 200rpm in the dark for 32h;
[0048] (3) After the reaction is completed, centrifuge the cells at 4°C and 50×100 rpm to collect the reaction solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com