A kind of vaccine composition and its preparation method and application
A vaccine composition, the technology of Haemophilus suis, applied in the field of vaccine composition and its preparation and application, can solve the problems of maternal antibody interference, not reducing the immune effect, etc., and achieve the effect of controllable quality and good immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
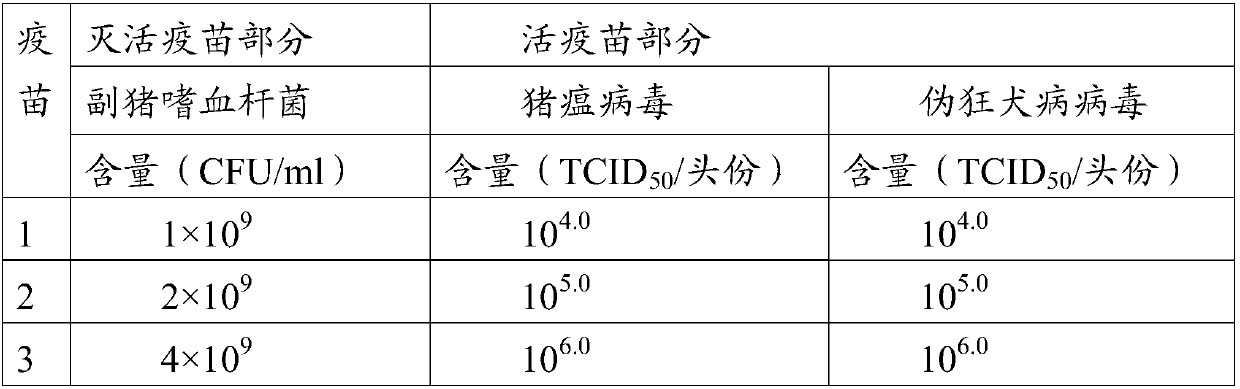
[0046] Embodiment 1 Preparation of Anti-Swine Fever Virus, Porcine Pseudorabies Virus and Haemophilus parasuis Vaccine Composition
[0047] 1. Preparation and testing of CSFV antigen solution
[0048] (1) Treat ST cells (purchased from ATCC) (American typeculture collection, American Type Culture Collection) that have grown into a good monolayer with high sensitivity to classical swine fever virus with 0.125wt% trypsin and 0.03wt% EDTA (B Diaminetetraacetic acid) digestion solution was digested and dispersed, the cells were counted, and after inoculation at a suitable density, MEM containing 3wt%-5wt% FBS (fetal bovine serum) (purchased from PAA Company, batch number A15110-1462) was added ( Purchased from GIBCO Company, batch number 856833), at the same time add seed virus according to M.O.I. (multiplicity of infection) = 0.1-0.2 inoculation dose, and place in an incubator at 34-37°C for cultivation.
[0049] (2) After three days of cultivation, the first poisoning was carri...
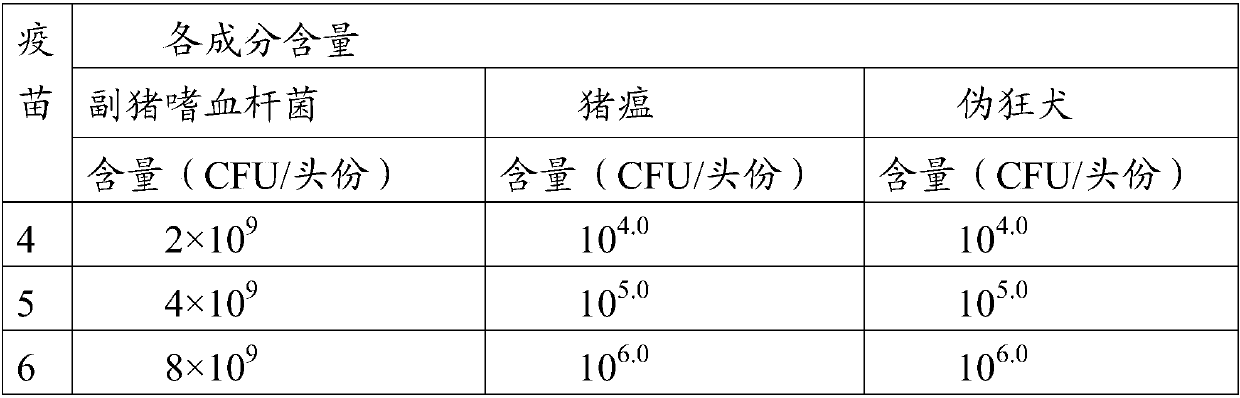
Embodiment 2
[0069] Example 2 Correlative inspection of classical swine fever virus, porcine pseudorabies virus and Haemophilus parasuis vaccine
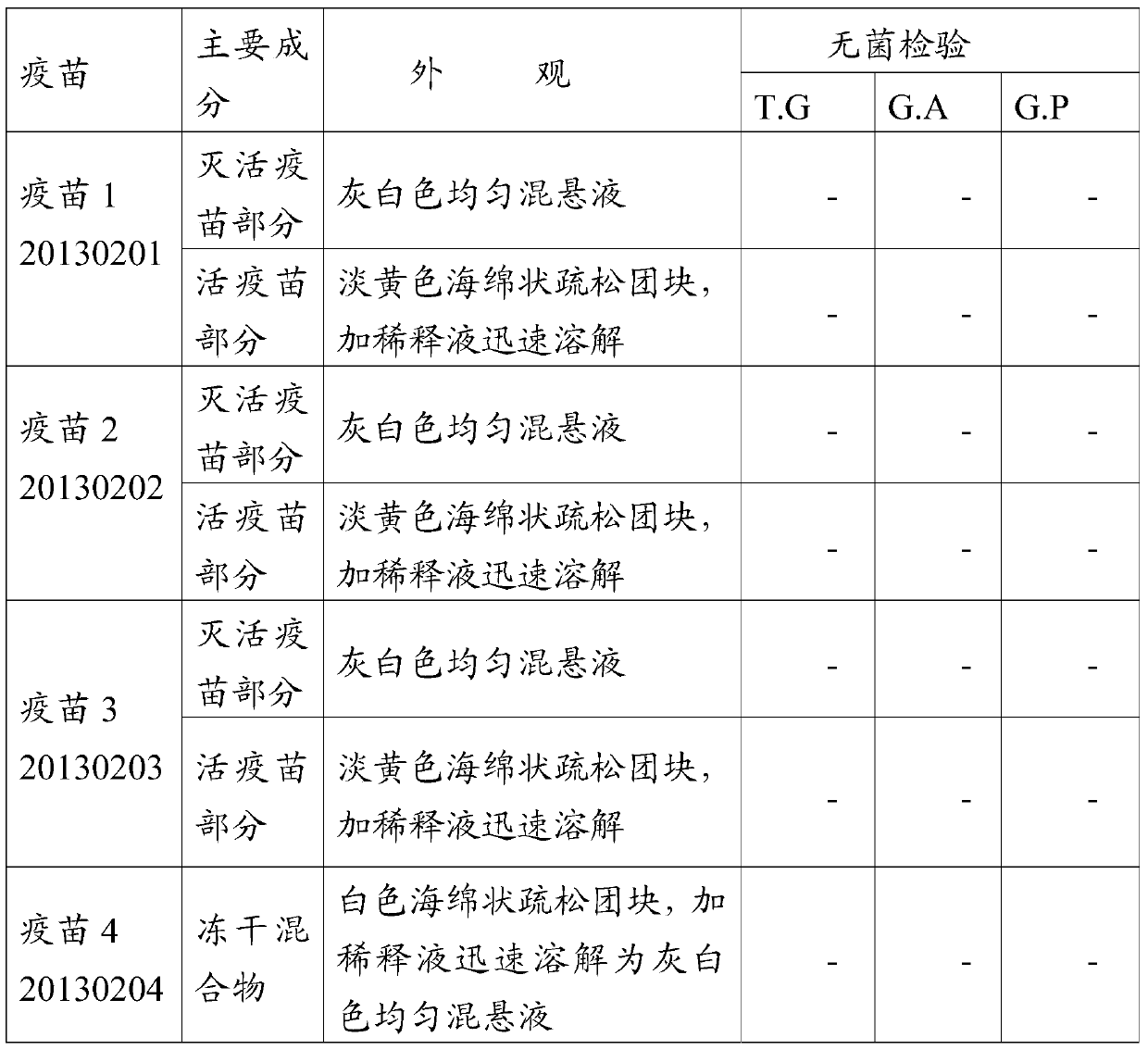
[0070] 1. Physical property test, sterility test
[0071] Vaccines 1 to 8 passed the physical property test and sterility test, and the detailed results are shown in Table 3:
[0072] Table 3 Vaccine physical properties test and sterility test
[0073]
[0074]
[0075] Note: T.G means thioglycolate medium, G.A means casease agar medium, G.P glucose-peptone medium; "-" means sterile growth.
[0076] 2 safety test
[0077] With 45 piglets negative for classical swine fever virus, porcine pseudorabies virus and Haemophilus parasuis antigen and antibody at the age of 2 to 3 weeks, 5 pigs in groups 1 to 3 were inoculated with 2 parts of inactivated vaccine partially diluted in each muscle 1 part of the sample to be tested from the live vaccine; 2 parts of freeze-dried diluted vaccine in each of the 5 heads of the 4th to 6th groups; the 5th ...
Embodiment 3
[0095] Embodiment 3 Application of swine fever, porcine pseudorabies and Haemophilus parasuis vaccine composition of the present invention
[0096] Trial 1: Comparative trial of vaccines
[0097] 1. Test materials The swine fever ELISA antibody detection kit was purchased from Beijing IDEXX Biotechnology Co., Ltd.; the porcine pseudorabies ELISA gB antibody detection kit was purchased from Beijing IDEXX Biotechnology Co., Ltd.
[0098] The vaccine batch number of the swine fever, porcine pseudorabies and Haemophilus parasuis vaccine of the present invention is several bottles of 20130202; the batch number of the live vaccine of swine fever produced by Guangdong Yongshun is 2012043, and the Haemophilus parasuis disease produced by Boehringer Ingelheim The batch number of live vaccine (z-1517) is 746-380, and the batch number of porcine pseudorabies live vaccine produced by Boehringer Ingelheim is 195A-23B.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com