2,3,5-Trifluoro-4-difluoro(3,4,5-trifluorophenylol)methyl-benzaldehyde, its synthetic method and its application in preparation of liquid crystal compound
A technology of trifluorophenol and compounds, which is applied in the application field of preparing liquid crystal compounds, can solve the problems of unsuitability for industrial production, high raw material prices, difficult purification of products, etc., and achieve easy industrial production, low purchase cost and good yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060]
[0061] 1) Synthesis of Compound 2
[0062] Weigh 120g of zinc powder and add it into a 2L three-necked bottle, then add 60g of compound 1, add 1200mL of ammonia water into the bottle, and mechanically stir for 40h.
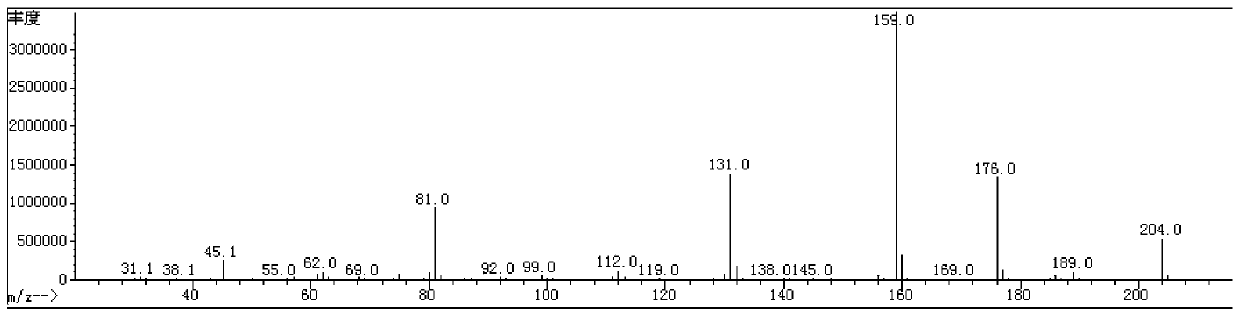
[0063] The reaction solution was poured into ice water, adjusted to pH=1-2 with concentrated hydrochloric acid, extracted with dichloromethane and recrystallized with ethyl acetate+petroleum ether to obtain 42.8g of white solid. Reaction with boron trifluoride ether and ethanol proved to be compound 2 with a purity of 95%. MS m / z: 204 (M + ), yield: 78.7%. For the MS diagram of compound 2, see figure 1 .
[0064] 2) Synthesis of Compound 3
[0065] Weigh 49g of zinc chloride and add it to a 1L single-necked bottle, add 500mL of tetrahydrofuran, add 27.4g of sodium borohydride in batches, stir at room temperature for 2 hours, slowly add 42.8g of compound 2 dropwise, slowly raise the temperature to 72°C, and react after 3 hours completely.
[0066...
Embodiment 2
[0094]
[0095] Weigh 27.5g of compound 8 synthesized in Example 1, 12g of ethyl propylene glycol, and 1.3g of p-toluenesulfonic acid into a 500mL single-necked bottle, add 200mL of toluene, and reflux at 135°C for 5h. TLC monitors that the reaction is complete.
[0096] After the reaction of the raw materials was completed, it was quenched with water, and the organic layer was separated, extracted twice with ethyl acetate, washed twice with water and once with saturated brine, dried over anhydrous sodium sulfate, and filtered. The organic phase was spin-dried, and petroleum ether was roughly passed through the column, and then distilled under reduced pressure to obtain 12 g of white crystal compound 11, GC: 99%, yield: 35.2%.
[0097] Liquid crystal properties of compound 11:
[0098] Δn: 0.0795;
[0099] Δε: 19.4;
[0100] Cp: -29.2.
Embodiment 3
[0102]
[0103] Weigh 27.5g of compound 8 synthesized in Example 1, 14g of 2-ethoxy-1,3-propanediol, and 1.3g of p-toluenesulfonic acid, add them to a 500mL single-necked bottle, add 200mL of toluene, and reflux at 135°C for 5h , TLC monitors that the reaction is complete.
[0104] After the reaction of the raw materials was completed, the mixture was quenched with water, and the organic layer was separated, extracted twice with ethyl acetate, washed twice with water and once with saturated brine, dried over anhydrous sodium sulfate, and filtered. The organic phase was spin-dried, and petroleum ether was roughly passed through the column, and then distilled under reduced pressure to obtain 14 g of white crystal compound 12, GC: 99.2%, yield: 39.5%.
[0105] Liquid crystal properties of compound 12:
[0106] Δn: 0.0812;
[0107] Δε: 22.2;
[0108] Cp: -15.4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com