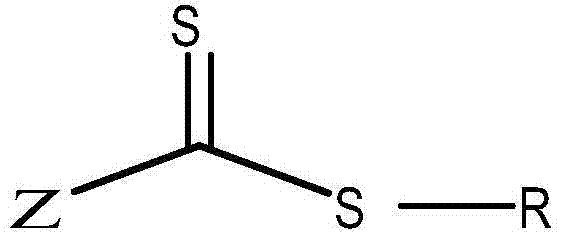
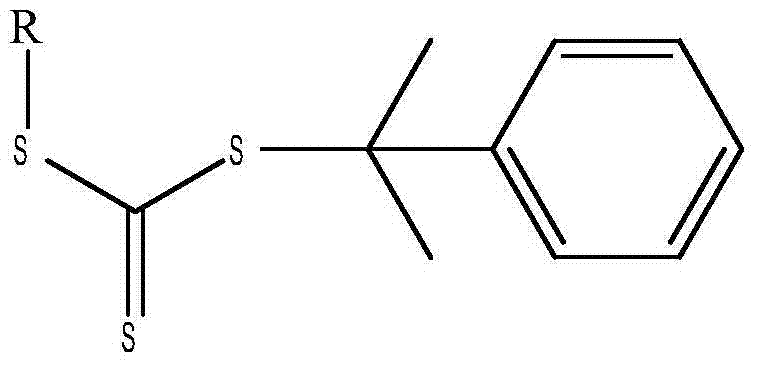
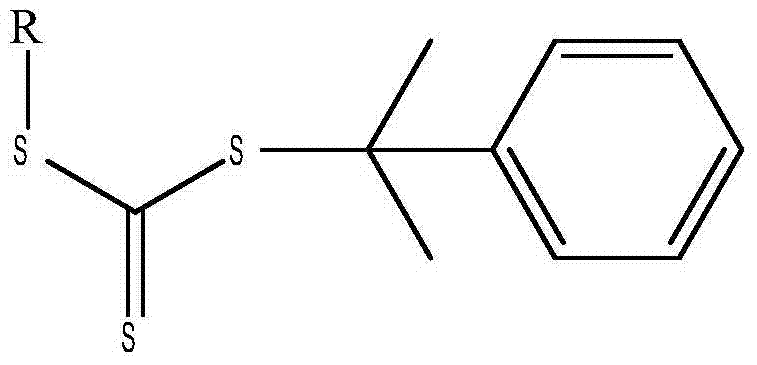
A reversible addition-fragmentation chain transfer agent, and preparation and applications thereof
A chain transfer reagent and addition-fragmentation technology, applied in organic chemistry and other fields, can solve problems such as the difficulty of purification restricting the large-scale use of RAFT polymerization, harsh preparation reaction conditions, and the influence of RAFT reagents, and achieve strong reversible addition-fragmentation capabilities , good control, low price effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] At 0°C, add 6.2g of ethanethiol dropwise to the acetone suspension containing 8.0g of potassium hydride. After the dropwise addition, add 15.2g of carbon disulfide to the suspension. The molar ratio of the three components is 0.1 :0.2:0.2. Stir at 0°C for 0.5h. Then filter, wash the filter residue with acetone for 3 times, add acetonitrile to dissolve, filter, and obtain the filtrate to dry by rotary evaporation to obtain potassium ethyl trithiocarbonate.
[0021] Disperse 8.8 g of the obtained potassium ethyl trithiocarbonate in the ether solution, add dropwise 3.9 g of 2-chloro-2-phenylpropane, the molar ratio between the two is 0.05:0.025, at 10°C After stirring for 6 h, the obtained product was washed with water for 5 times, subjected to liquid separation treatment, and the organic solvent was removed to obtain cumyl ethyl trithiocarbonate with a yield of about 93%.
Embodiment 2
[0023] At 5°C, add 9.0g of butanethiol dropwise to the tetrahydrofuran suspension containing 6.0g of potassium hydride. After the addition is complete, add 15.2g of carbon disulfide to the suspension. The molar ratio of the three components is 0.1 :0.15:0.2. Stir at 5 °C for 1 h. Then it was filtered, and the filter residue was washed three times with tetrahydrofuran, dissolved in acetonitrile, filtered, and the obtained filtrate was dried by rotary evaporation to obtain butyl potassium trithiocarbonate.
[0024] Disperse 10.2 g of the obtained butyl potassium trithiocarbonate in an acetone solution, add dropwise 6.0 g of 2-bromo-2-phenylpropane, the molar ratio between the two is 0.05:0.03, and at 20°C After stirring for 4 hours, the obtained product was washed with water three times, subjected to liquid separation treatment, and the organic solvent was removed to obtain cumyl butyl trithiocarbonate with a yield of about 95%.
Embodiment 3
[0026] At 2°C, add 11.8g of hexanethiol dropwise to the suspension in tetrahydrofuran containing 3.0g of sodium hydride. After the addition is complete, add 11.4g of carbon disulfide to the suspension. The molar ratio of the three components is 0.1 :0.125:0.15. Stir at 2°C for 1.5h. Then filter, wash the filter residue three times with tetrahydrofuran, add acetonitrile to dissolve, filter, and obtain the filtrate by rotary evaporation and drying to obtain hexyl sodium trithiocarbonate.
[0027] Disperse 10.8 g of the obtained hexyl sodium trithiocarbonate in an acetone solution, add dropwise 5.4 g of 2-chloro-2-phenylpropane, the molar ratio between the two is 0.05:0.035, and stir at 50°C After 1 h, the obtained product was washed with water three times, subjected to liquid separation treatment, and the organic solvent was removed to obtain cumyl hexyl trithiocarbonate with a yield of about 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com